Methadone pharmacogenetics in vitro and in vivo: Metabolism by CYP2B6 polymorphic variants and genetic variability in paediatric disposition
- PMID: 35538637
- PMCID: PMC10908252
- DOI: 10.1111/bcp.15393
Methadone pharmacogenetics in vitro and in vivo: Metabolism by CYP2B6 polymorphic variants and genetic variability in paediatric disposition
Abstract
Aims: Methadone metabolism and clearance are determined principally by polymorphic cytochrome P4502B6 (CYP2B6). Some CYP2B6 allelic variants affect methadone metabolism in vitro and disposition in vivo. We assessed methadone metabolism by CYP2B6 minor variants in vitro. We also assessed the influence of CYP2B6 variants, and P450 oxidoreductase (POR) and CYP2C19 variants, on methadone clearance in surgical patients in vivo.
Methods: CYP2B6 and P450 oxidoreductase variants were coexpressed with cytochrome b5 . The metabolism of methadone racemate and enantiomers was measured at therapeutic concentrations and intrinsic clearances were determined. Adolescents receiving methadone for surgery were genotyped for CYP2B6, CYP2C19 and POR, and methadone clearance and metabolite formation clearance were determined.
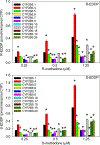
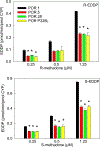
Results: In vitro, CYP2B6.4 was more active than wild-type CYP2B6.1. CYPs 2B6.5, 2B6.6, 2B6.7, 2B6.9, 2B6.17, 2B6.19 and 2B6.26 were less active. CYPs 2B6.16 and 2B6.18 were inactive. CYP2B6.1 expressed with POR variants POR.28, POR.5 and P228L had lower rates of methadone metabolism than wild-type reductase. In vivo, methadone clinical clearance decreased linearly with the number of CYP2B6 slow metabolizer alleles, but was not different in CYP2C19 slow or rapid metabolizer phenotypes, or in carriers of the POR*28 allele.
Conclusions: Several CYP2B6 and POR variants were slow metabolizers of methadone in vitro. Polymorphisms in CYP2B6, but not CYP2C19 or P450 reductase, affected methadone clearance in vivo. CYP2B6 polymorphisms 516G>T and 983T>C code for canonical loss of function variants and should be assessed when considering genetic influences on clinical methadone disposition. These complementary translational in vitro and in vivo results inform on pharmacogenetic variability affecting methadone disposition in patients.
Keywords: CYP2B6; CYP2B6 polymorphisms; cytochrome P4502B6; methadone.
© 2022 British Pharmacological Society.
Figures





Similar articles
-
Pharmacogenetic Influence on Stereoselective Steady-State Disposition of Bupropion.Drug Metab Dispos. 2024 Apr 16;52(5):455-466. doi: 10.1124/dmd.124.001697. Drug Metab Dispos. 2024. PMID: 38467432 Free PMC article. Clinical Trial.
-
Stereoselective Ketamine Metabolism by Genetic Variants of Cytochrome P450 CYP2B6 and Cytochrome P450 Oxidoreductase.Anesthesiology. 2018 Oct;129(4):756-768. doi: 10.1097/ALN.0000000000002371. Anesthesiology. 2018. PMID: 30085944
-
Differences in Methadone Metabolism by CYP2B6 Variants.Drug Metab Dispos. 2015 Jul;43(7):994-1001. doi: 10.1124/dmd.115.064352. Epub 2015 Apr 20. Drug Metab Dispos. 2015. PMID: 25897175 Free PMC article.
-
Efavirenz Metabolism: Influence of Polymorphic CYP2B6 Variants and Stereochemistry.Drug Metab Dispos. 2019 Oct;47(10):1195-1205. doi: 10.1124/dmd.119.086348. Epub 2019 Jul 19. Drug Metab Dispos. 2019. PMID: 31324697 Free PMC article. Review.
-
Effects of cytochrome P450 single nucleotide polymorphisms on methadone metabolism and pharmacodynamics.Biochem Pharmacol. 2018 Jul;153:196-204. doi: 10.1016/j.bcp.2018.02.020. Epub 2018 Feb 16. Biochem Pharmacol. 2018. PMID: 29458047 Free PMC article. Review.
Cited by
-
Pharmacogenetic Influence on Stereoselective Steady-State Disposition of Bupropion.Drug Metab Dispos. 2024 Apr 16;52(5):455-466. doi: 10.1124/dmd.124.001697. Drug Metab Dispos. 2024. PMID: 38467432 Free PMC article. Clinical Trial.
-
The Relevance of Pharmacokinetic Biomarkers in Response to Methadone Treatment: A Systematic Review.Pharmaceuticals (Basel). 2025 Apr 25;18(5):623. doi: 10.3390/ph18050623. Pharmaceuticals (Basel). 2025. PMID: 40430443 Free PMC article. Review.
-
Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2B6 Genotype and Methadone Therapy.Clin Pharmacol Ther. 2024 Oct;116(4):932-938. doi: 10.1002/cpt.3338. Epub 2024 Jun 11. Clin Pharmacol Ther. 2024. PMID: 38863207 Free PMC article.
References
-
- Hanna V, Senderovich H. Methadone in pain management: A systematic review. J Pain. 2021;22:233–45. - PubMed
-
- Oesterle TS, Thusius NJ, Rummans TA, Gold MS. Medication-assisted treatment for opioid-use disorder. Mayo Clin Proc. 2019;94:2072–86. - PubMed
-
- Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6. - PubMed
-
- Murphy GS, Szokol JW. Intraoperative methadone in surgical patients: A review of clinical investigations. Anesthesiology. 2019;131:678–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

