Biofunctionalization of Graphene-Based FET Sensors through Heterobifunctional Nanoscaffolds: Technology Validation toward Rapid COVID-19 Diagnostics and Monitoring
- PMID: 35538925
- PMCID: PMC9073996
- DOI: 10.1002/admi.202102526
Biofunctionalization of Graphene-Based FET Sensors through Heterobifunctional Nanoscaffolds: Technology Validation toward Rapid COVID-19 Diagnostics and Monitoring
Abstract
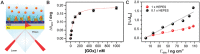
The biofunctionalization of graphene field-effect transistors (GFETs) through vinylsulfonated-polyethyleneimine nanoscaffold is presented for enhanced biosensing of severe acute respiratory-related coronavirus 2 (SARS-CoV-2) spike protein and human ferritin, two targets of great importance for the rapid diagnostic and monitoring of individuals with COVID-19. The heterobifunctional nanoscaffold enables covalent immobilization of binding proteins and antifouling polymers while the whole architecture is attached to graphene by multivalent π-π interactions. First, to optimize the sensing platform, concanavalin A is employed for glycoprotein detection. Then, monoclonal antibodies specific against SARS-CoV-2 spike protein and human ferritin are anchored, yielding biosensors with limit of detections of 0.74 and 0.23 nm, and apparent affinity constants ( ) of 6.7 and 8.8 nm, respectively. Both biosensing platforms show good specificity, fast time response, and wide dynamic range (0.1-100 nm). Moreover, SARS-CoV-2 spike protein is also detected in spiked nasopharyngeal swab samples. To rigorously validate this biosensing technology, the GFET response is matched with surface plasmon resonance measurements, exhibiting linear correlations (from 2 to 100 ng cm-2) and good agreement in terms of K D values. Finally, the performance of the biosensors fabricated through the nanoscaffold strategy is compared with those obtained through the widely employed monopyrene approach, showing enhanced sensitivity.
Keywords: COVID‐19; ferritin; field‐effect transistors; graphene; severe acute respiratory‐related coronavirus 2; spike protein; surface plasmon resonance.
© 2022 Wiley‐VCH GmbH.
Conflict of interest statement
E.P., W.A.M., and O.A. are scientific advisors of GISENS BIOTECH through a contract between UNLP, CONICET, and GISENS BIOTECH. A.L.C. and J.M.P. are recently or presently employed by GISENS BIOTECH. The other authors declare no conflict of interest.
Figures





References
-
- Adam D. C., Wu P., Wong J. Y., Lau E. H. Y., Tsang T. K., Cauchemez S., Leung G. M., Cowling B. J., Nat. Med. 2020, 26, 1714. - PubMed
-
- SARS‐CoV‐2 Antigen‐Detecting Rapid Diagnostic Tests: An Implementation Guide, World Health Organization, Geneva, 2020.
-
- Shan D., Johnson J. M., Fernandes S. C., Suib H., Hwang S., Wuelfing D., Mendes M., Holdridge M., Burke E. M., Beauregard K., Zhang Y., Cleary M., Xu S., Yao X., Patel P. P., Plavina T., Wilson D. H., Chang L., Kaiser K. M., Nattermann J., Schmidt S. V., Latz E., Hrusovsky K., Mattoon D., Ball A. J., Nat. Commun. 2021, 12, 1931. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
