Highly stable mesoporous silica nanospheres embedded with FeCo/graphitic shell nanocrystals as magnetically recyclable multifunctional adsorbents for wastewater treatment
- PMID: 35538962
- PMCID: PMC9077014
- DOI: 10.1039/c7ra12240c
Highly stable mesoporous silica nanospheres embedded with FeCo/graphitic shell nanocrystals as magnetically recyclable multifunctional adsorbents for wastewater treatment
Abstract
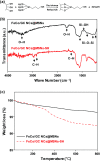
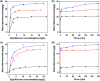
Highly stable and magnetically separable mesoporous silica nanospheres (MSNs) embedded with 4.6 ± 0.8 nm FeCo/graphitic carbon shell nanocrystals (FeCo/GC NCs@MSNs) were synthesized by thermal decomposition of metal precursors in MSNs and subsequent methane CVD. The FeCo/GC NCs@MSNs had a high specific surface area (442 m2 g-1), large pore volume (0.65 cm3 g-1), and tunable size (65 nm, 130 nm, and 270 nm). Despite the low magnetic metal content (8.35 wt%), the FeCo/GC NCs@MSNs had a sufficiently high saturation magnetization (17.1 emu g-1). This is due to the superior magnetic properties of the FeCo/GC NCs, which also enable fast magnetic separation of the nanospheres. The graphitic carbon shell on the FeCo NCs not only protects the alloy core against oxidation and acid etching in 35% HCl(aq), but also facilitates non-covalent, hydrophobic interactions with the hydrocarbon chains of organic dyes such as methyl orange and methylene blue. Surface functionalization of the FeCo/GC NCs@MSNs with thiol groups provides efficient capacity for binding with Hg2+ ions. We have shown that the thiol-functionalized FeCo/GC NCs@MSNs (FeCo/GC NCs@MSNs-SH) work as multifunctional adsorbents for organic dyes (target organic pollutants) and Hg2+ ions (target inorganic pollutant). We also demonstrated that the FeCo/GC NCs@MSNs-SH are excellent recyclable adsorbents for methyl orange.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Au nanoparticle@hollow mesoporous carbon with FeCo/graphitic shell nanoparticls as a magnetically recyclable yolk-shell nanocatalyst for catalytic reduction of nitroaromatics.Sci Rep. 2018 May 10;8(1):7469. doi: 10.1038/s41598-018-25795-w. Sci Rep. 2018. PMID: 29748617 Free PMC article.
-
Propargylic substitution reactions with various nucleophilic compounds using efficient and recyclable mesoporous silica spheres embedded with FeCo/graphitic shell nanocrystals.Nanoscale Res Lett. 2015 Jan 23;10:2. doi: 10.1186/1556-276X-10-2. eCollection 2015. Nanoscale Res Lett. 2015. PMID: 25852304 Free PMC article.
-
A Highly Stable and Magnetically Recyclable Nanocatalyst System: Mesoporous Silica Spheres Embedded with FeCo/Graphitic Shell Magnetic Nanoparticles and Pt Nanocatalysts.Chem Asian J. 2015 Dec;10(12):2755-61. doi: 10.1002/asia.201500773. Epub 2015 Sep 9. Chem Asian J. 2015. PMID: 26312570
-
Recent Advances in the Synthesis, Surface Modifications and Applications of Core-Shell Magnetic Mesoporous Silica Nanospheres.Chem Asian J. 2020 Apr 17;15(8):1248-1265. doi: 10.1002/asia.202000045. Epub 2020 Mar 18. Chem Asian J. 2020. PMID: 32083794 Review.
-
Inorganic Nanocrystals Functionalized Mesoporous Silica Nanoparticles: Fabrication and Enhanced Bio-applications.Front Chem. 2017 Dec 13;5:118. doi: 10.3389/fchem.2017.00118. eCollection 2017. Front Chem. 2017. PMID: 29326923 Free PMC article. Review.
Cited by
-
Au nanoparticle@hollow mesoporous carbon with FeCo/graphitic shell nanoparticls as a magnetically recyclable yolk-shell nanocatalyst for catalytic reduction of nitroaromatics.Sci Rep. 2018 May 10;8(1):7469. doi: 10.1038/s41598-018-25795-w. Sci Rep. 2018. PMID: 29748617 Free PMC article.
-
Development and application of novel bio-magnetic membrane capsules for the removal of the cationic dye malachite green in wastewater treatment.RSC Adv. 2019 Jan 28;9(7):3625-3646. doi: 10.1039/c8ra09275c. eCollection 2019 Jan 25. RSC Adv. 2019. PMID: 35518114 Free PMC article.
-
Recent progress in the applications of silica-based nanoparticles.RSC Adv. 2022 May 6;12(22):13706-13726. doi: 10.1039/d2ra01587k. eCollection 2022 May 5. RSC Adv. 2022. PMID: 35530394 Free PMC article. Review.
-
Maleic Anhydride Cross-Linked β-Cyclodextrin-Conjugated Magnetic Nanoadsorbent: An Ecofriendly Approach for Simultaneous Adsorption of Hydrophilic and Hydrophobic Dyes.ACS Omega. 2019 Jul 10;4(7):11993-12003. doi: 10.1021/acsomega.9b00881. eCollection 2019 Jul 31. ACS Omega. 2019. PMID: 31460311 Free PMC article.
-
Synthesis of MoS2 nanosheets for mercury speciation analysis by HPLC-UV-HG-AFS.RSC Adv. 2018 May 18;8(33):18364-18371. doi: 10.1039/c8ra01891j. eCollection 2018 May 17. RSC Adv. 2018. PMID: 35541115 Free PMC article.
References
-
- Gupta V. K. Moradi O. Tyagi I. Agarwal S. Sadegh H. Shahryari-Ghoshekandi R. Makhlouf A. S. H. Goodarzi M. Garshasbi A. Crit. Rev. Environ. Sci. Technol. 2016;46:93–118. doi: 10.1080/10643389.2015.1061874. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

