Synthesis, chiroptical properties, and self-assembled nanoparticles of chiral conjugated polymers based on optically stable helical aromatic esters
- PMID: 35538983
- PMCID: PMC9076947
- DOI: 10.1039/c7ra12652b
Synthesis, chiroptical properties, and self-assembled nanoparticles of chiral conjugated polymers based on optically stable helical aromatic esters
Abstract
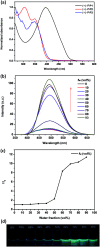
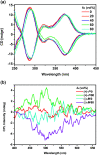
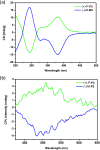
By Suzuki coupling reaction, three pairs of chiral conjugated polymers with optically stable helical aromatic ester subunits as the main-chain were designed and synthesized. Polymers (+)-P-P1 and (-)-M-P1, (+)-P-P2 and (-)-M-P2 showed strong fluorescence emission, strong mirror image CD and circularly polarized luminescence (CPL) signals in THF. For polymers (+)-P-P3 and (-)-M-P3, containing the tetraphenylethene (TPE) moiety, they not only showed obvious aggregation induced enhancement emission (AIEE), but also exhibited mirror image CD signals and aggregation-induced enhancement CPL signals in THF-water mixtures. Moreover, (+)-P-P3 and (-)-M-P3 could also form chiral nanoparticles by solvent evaporation induced self-assembly. Interestingly, it was further found that the size of the nanoparticles could be controlled by the changing of THF/water ratio, and their CPL properties were also shown.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Chiral Nanoparticles with Full-Color and White CPL Properties Based on Optically Stable Helical Aromatic Imide Enantiomers.ACS Appl Mater Interfaces. 2018 Mar 7;10(9):8225-8230. doi: 10.1021/acsami.8b00341. Epub 2018 Feb 23. ACS Appl Mater Interfaces. 2018. PMID: 29436220
-
Inverted Circularly Polarized Luminescence Behavior Induced by Helical Nanofibers through Chiral Co-Assembly from Achiral Liquid Crystal Polymers and Chiral Inducers.ACS Nano. 2022 Feb 22;16(2):3173-3181. doi: 10.1021/acsnano.1c11011. Epub 2022 Feb 10. ACS Nano. 2022. PMID: 35142484
-
Helically assembled π-conjugated polymers with circularly polarized luminescence.Sci Technol Adv Mater. 2014 Aug 21;15(4):044203. doi: 10.1088/1468-6996/15/4/044203. eCollection 2014 Aug. Sci Technol Adv Mater. 2014. PMID: 27877698 Free PMC article. Review.
-
Helix-Induced Asymmetric Self-Assembly of π-Conjugated Block Copolymers: From Controlled Syntheses to Distinct Properties.Acc Chem Res. 2023 Nov 7;56(21):2954-2967. doi: 10.1021/acs.accounts.3c00425. Epub 2023 Oct 18. Acc Chem Res. 2023. PMID: 37852202
-
Polymers with Circularly Polarized Luminescent Properties: Design, Synthesis, and Prospects.Chempluschem. 2024 May;89(5):e202300481. doi: 10.1002/cplu.202300481. Epub 2023 Dec 7. Chempluschem. 2024. PMID: 37955194 Review.
Cited by
-
Dual-Mode Induction of Tunable Circularly Polarized Luminescence from Chiral Metal-Organic Frameworks.Research (Wash D C). 2020 Jan 23;2020:6452123. doi: 10.34133/2020/6452123. eCollection 2020. Research (Wash D C). 2020. PMID: 32025662 Free PMC article.
-
Aggregation-Induced Emission Properties in Fully π-Conjugated Polymers, Dendrimers, and Oligomers.Polymers (Basel). 2021 Jan 9;13(2):213. doi: 10.3390/polym13020213. Polymers (Basel). 2021. PMID: 33435293 Free PMC article. Review.
-
Chiral assembly of organic luminogens with aggregation-induced emission.Chem Sci. 2021 Jun 10;13(3):611-632. doi: 10.1039/d1sc02305e. eCollection 2022 Jan 19. Chem Sci. 2021. PMID: 35173927 Free PMC article. Review.
-
Solid-state AIEnh-circularly polarised luminescence of chiral perylene diimide fluorophores.RSC Adv. 2019 Jan 14;9(4):1976-1981. doi: 10.1039/c8ra09785b. eCollection 2019 Jan 14. RSC Adv. 2019. PMID: 35516153 Free PMC article.
-
Efficient and Recyclable RuCl3 ⋅ 3H2O Catalyst Modified with Ionic Diphosphine for the Alkoxycarbonylation of Aryl Halides.ChemistryOpen. 2019 Jan 23;8(2):166-172. doi: 10.1002/open.201800266. eCollection 2019 Feb. ChemistryOpen. 2019. PMID: 30740291 Free PMC article.
References
-
- Steiner F. Lupton J. M. Vogelsang J. J. Am. Chem. Soc. 2017;139:9787–9790. doi: 10.1021/jacs.7b04619. - DOI - PubMed
- Zhang Z. G. Yang Y. Yao J. Xue L. Chen S. Li X. Morrison W. Yang C. Li Y. Angew. Chem., Int. Ed. 2017;56:13503–13507. doi: 10.1002/anie.201707678. - DOI - PubMed
- Boroumand F. A. Fry P. W. Lidzey D. G. Nano Lett. 2005;5:67–71. doi: 10.1021/nl048382k. - DOI - PubMed
- Vohra V. Giovanella U. Tubino R. Murata H. Botta C. ACS Nano. 2011;5:5572–5578. doi: 10.1021/nn201029c. - DOI - PubMed
- Vohra V. Giovanella U. Tubino R. Murata H. Botta C. J. Am. Chem. Soc. 2017;139:11666–11669. doi: 10.1021/jacs.7b05025. - DOI - PubMed
- Lv Y. Liu P. Ding H. Wu Y. Yan Y. Liu H. Wang X. Huang F. Zhao Y. Tian Z. ACS Appl. Mater. Interfaces. 2015;7:20640–20648. doi: 10.1021/acsami.5b05150. - DOI - PubMed
- Feng X. Lv F. Liu L. Tang H. Xing C. Yang Q. Wang S. ACS Appl. Mater. Interfaces. 2010;2:2429–2435. doi: 10.1021/am100435k. - DOI - PubMed
-
- Pecher J. Mecking S. Chem. Rev. 2010;110:6260–6279. doi: 10.1021/cr100132y. - DOI - PubMed
- Jiang Y. Upputuri P. K. Xie C. Lyu Y. Zhang L. Xiong Q. Pramanic M. Pu K. Nano Lett. 2017;17:4964–4969. doi: 10.1021/acs.nanolett.7b02106. - DOI - PubMed
- Vithanage D. A. Kanibolotsky A. L. Rajbhandari S. Manousiadis P. P. Sajjad M. T. Chun H. Faulkner G. E. O'Brien D. C. Skabara P. J. Samuel I. D. W. Turnbull G. A. J. Mater. Chem. C. 2017;5:8916–8920. doi: 10.1039/C7TC03787B. - DOI
- Lidster B. J. Kumar D. R. Spring A. M. Yu C.-Y. Turner M. L. Polym. Chem. 2016;7:5544–5551. doi: 10.1039/C6PY01186A. - DOI - PubMed
-
- Zhang S. Sheng Y. Wei G. Quan Y. Cheng Y. Zhu C. Polym. Chem. 2015;6:2416–2422. doi: 10.1039/C4PY01689K. - DOI
- Wei J. Zhang X. Zhao Y. Li R. Macromol. Chem. Phys. 2013;214:2232–2238.
- Meskers S. C. J. Peeters E. Langeveld-Voss B. M. W. Janssen R. A. J. Adv. Mater. 2000;12:589–594. doi: 10.1002/(SICI)1521-4095(200004)12:8<589::AID-ADMA589>3.0.CO;2-C. - DOI
- Peeters E. Christiaans M. P. T. Janssen R. A. J. Schoo H. F. M. Dekkers H. P. J. M. Meijer E. W. J. Am. Chem. Soc. 1997;119:9909–9910. doi: 10.1021/ja971912c. - DOI
- Hayasaka H. Miyashita T. Tamura K. Akagi K. Adv. Funct. Mater. 2010;20:1243–1250. doi: 10.1002/adfm.200902059. - DOI
-
- Lim C.-K. Cho M. J. Singh A. Li Q. Kim W. J. Jee H. S. Fillman K. L. Carpenter S. H. Neidig M. L. Baev A. Swihart M. T. Prasad P. N. Nano Lett. 2016;16:5451–5455. doi: 10.1021/acs.nanolett.6b01874. - DOI - PubMed
- Hou J. Song F. Wang L. Wei G. Cheng Y. Zhu C. Macromolecules. 2012;45:7835–7842. doi: 10.1021/ma301553y. - DOI
- Shao H. Lockman J. W. Parquette J. R. J. Am. Chem. Soc. 2007;129:1884–1885. doi: 10.1021/ja068154n. - DOI - PubMed
- Ho R.-M. Li M.-C. Lin S.-C. Wang H.-F. Lee Y.-D. Hasegawa H. Thomas E. J. Am. Chem. Soc. 2012;134:10974–10986. doi: 10.1021/ja303513f. - DOI - PubMed
-
- Huang W.-S. Hu Q.-S. Zheng X.-F. Anderson J. Pu L. J. Am. Chem. Soc. 1997;119:4313–4314. doi: 10.1021/ja964285k. - DOI
- Hu Q.-S. Huang W.-S. Vitharana D. Zheng X.-F. Pu L. J. Am. Chem. Soc. 1997;119:12454–12464. doi: 10.1021/ja972623r. - DOI
- Pu L. Acc. Chem. Res. 2017;50:1032–1040. doi: 10.1021/acs.accounts.7b00036. - DOI - PubMed
- Zhang X. Wang C. Wang P. Du J. Zhang G. Pu L. Chem. Sci. 2016;7:3614–3620. doi: 10.1039/C6SC00266H. - DOI - PMC - PubMed
- Huang W.-S. Hu Q.-S. Pu L. J. Org. Chem. 1999;64:7940–7956. doi: 10.1021/jo990992v. - DOI - PubMed
LinkOut - more resources
Full Text Sources

