Synthesis, chiroptical properties, and self-assembled nanoparticles of chiral conjugated polymers based on optically stable helical aromatic esters
- PMID: 35538983
- PMCID: PMC9076947
- DOI: 10.1039/c7ra12652b
Synthesis, chiroptical properties, and self-assembled nanoparticles of chiral conjugated polymers based on optically stable helical aromatic esters
Abstract
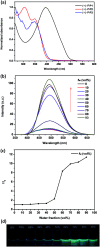
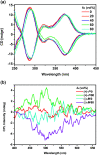
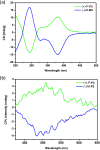
By Suzuki coupling reaction, three pairs of chiral conjugated polymers with optically stable helical aromatic ester subunits as the main-chain were designed and synthesized. Polymers (+)-P-P1 and (-)-M-P1, (+)-P-P2 and (-)-M-P2 showed strong fluorescence emission, strong mirror image CD and circularly polarized luminescence (CPL) signals in THF. For polymers (+)-P-P3 and (-)-M-P3, containing the tetraphenylethene (TPE) moiety, they not only showed obvious aggregation induced enhancement emission (AIEE), but also exhibited mirror image CD signals and aggregation-induced enhancement CPL signals in THF-water mixtures. Moreover, (+)-P-P3 and (-)-M-P3 could also form chiral nanoparticles by solvent evaporation induced self-assembly. Interestingly, it was further found that the size of the nanoparticles could be controlled by the changing of THF/water ratio, and their CPL properties were also shown.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Steiner F. Lupton J. M. Vogelsang J. J. Am. Chem. Soc. 2017;139:9787–9790. doi: 10.1021/jacs.7b04619. - DOI - PubMed
- Zhang Z. G. Yang Y. Yao J. Xue L. Chen S. Li X. Morrison W. Yang C. Li Y. Angew. Chem., Int. Ed. 2017;56:13503–13507. doi: 10.1002/anie.201707678. - DOI - PubMed
- Boroumand F. A. Fry P. W. Lidzey D. G. Nano Lett. 2005;5:67–71. doi: 10.1021/nl048382k. - DOI - PubMed
- Vohra V. Giovanella U. Tubino R. Murata H. Botta C. ACS Nano. 2011;5:5572–5578. doi: 10.1021/nn201029c. - DOI - PubMed
- Vohra V. Giovanella U. Tubino R. Murata H. Botta C. J. Am. Chem. Soc. 2017;139:11666–11669. doi: 10.1021/jacs.7b05025. - DOI - PubMed
- Lv Y. Liu P. Ding H. Wu Y. Yan Y. Liu H. Wang X. Huang F. Zhao Y. Tian Z. ACS Appl. Mater. Interfaces. 2015;7:20640–20648. doi: 10.1021/acsami.5b05150. - DOI - PubMed
- Feng X. Lv F. Liu L. Tang H. Xing C. Yang Q. Wang S. ACS Appl. Mater. Interfaces. 2010;2:2429–2435. doi: 10.1021/am100435k. - DOI - PubMed
-
- Pecher J. Mecking S. Chem. Rev. 2010;110:6260–6279. doi: 10.1021/cr100132y. - DOI - PubMed
- Jiang Y. Upputuri P. K. Xie C. Lyu Y. Zhang L. Xiong Q. Pramanic M. Pu K. Nano Lett. 2017;17:4964–4969. doi: 10.1021/acs.nanolett.7b02106. - DOI - PubMed
- Vithanage D. A. Kanibolotsky A. L. Rajbhandari S. Manousiadis P. P. Sajjad M. T. Chun H. Faulkner G. E. O'Brien D. C. Skabara P. J. Samuel I. D. W. Turnbull G. A. J. Mater. Chem. C. 2017;5:8916–8920. doi: 10.1039/C7TC03787B. - DOI
- Lidster B. J. Kumar D. R. Spring A. M. Yu C.-Y. Turner M. L. Polym. Chem. 2016;7:5544–5551. doi: 10.1039/C6PY01186A. - DOI - PubMed
-
- Zhang S. Sheng Y. Wei G. Quan Y. Cheng Y. Zhu C. Polym. Chem. 2015;6:2416–2422. doi: 10.1039/C4PY01689K. - DOI
- Wei J. Zhang X. Zhao Y. Li R. Macromol. Chem. Phys. 2013;214:2232–2238.
- Meskers S. C. J. Peeters E. Langeveld-Voss B. M. W. Janssen R. A. J. Adv. Mater. 2000;12:589–594. doi: 10.1002/(SICI)1521-4095(200004)12:8<589::AID-ADMA589>3.0.CO;2-C. - DOI
- Peeters E. Christiaans M. P. T. Janssen R. A. J. Schoo H. F. M. Dekkers H. P. J. M. Meijer E. W. J. Am. Chem. Soc. 1997;119:9909–9910. doi: 10.1021/ja971912c. - DOI
- Hayasaka H. Miyashita T. Tamura K. Akagi K. Adv. Funct. Mater. 2010;20:1243–1250. doi: 10.1002/adfm.200902059. - DOI
-
- Lim C.-K. Cho M. J. Singh A. Li Q. Kim W. J. Jee H. S. Fillman K. L. Carpenter S. H. Neidig M. L. Baev A. Swihart M. T. Prasad P. N. Nano Lett. 2016;16:5451–5455. doi: 10.1021/acs.nanolett.6b01874. - DOI - PubMed
- Hou J. Song F. Wang L. Wei G. Cheng Y. Zhu C. Macromolecules. 2012;45:7835–7842. doi: 10.1021/ma301553y. - DOI
- Shao H. Lockman J. W. Parquette J. R. J. Am. Chem. Soc. 2007;129:1884–1885. doi: 10.1021/ja068154n. - DOI - PubMed
- Ho R.-M. Li M.-C. Lin S.-C. Wang H.-F. Lee Y.-D. Hasegawa H. Thomas E. J. Am. Chem. Soc. 2012;134:10974–10986. doi: 10.1021/ja303513f. - DOI - PubMed
-
- Huang W.-S. Hu Q.-S. Zheng X.-F. Anderson J. Pu L. J. Am. Chem. Soc. 1997;119:4313–4314. doi: 10.1021/ja964285k. - DOI
- Hu Q.-S. Huang W.-S. Vitharana D. Zheng X.-F. Pu L. J. Am. Chem. Soc. 1997;119:12454–12464. doi: 10.1021/ja972623r. - DOI
- Pu L. Acc. Chem. Res. 2017;50:1032–1040. doi: 10.1021/acs.accounts.7b00036. - DOI - PubMed
- Zhang X. Wang C. Wang P. Du J. Zhang G. Pu L. Chem. Sci. 2016;7:3614–3620. doi: 10.1039/C6SC00266H. - DOI - PMC - PubMed
- Huang W.-S. Hu Q.-S. Pu L. J. Org. Chem. 1999;64:7940–7956. doi: 10.1021/jo990992v. - DOI - PubMed
LinkOut - more resources
Full Text Sources

