Chemical reaction-transport model of oxidized diethylzinc based on quantum mechanics and computational fluid dynamics approaches
- PMID: 35538984
- PMCID: PMC9077139
- DOI: 10.1039/c7ra11534b
Chemical reaction-transport model of oxidized diethylzinc based on quantum mechanics and computational fluid dynamics approaches
Abstract
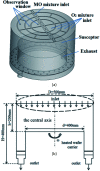
We developed and studied a chemical reaction-transport model for the production of zinc oxide (ZnO) with diethylzinc (DEZn) and oxygen (O2). It was confirmed that a large number of ZnO particles were generated during the growth process by testing the internal particles of the cavity by X-ray diffraction. The formation of Zn3O3 in the gas phase reaction was simulated using density functional theory, and the effect of nucleation and formation of nanoparticles on the growth of the films was revealed. We also speculate that the adsorption of Zn-containing gas on the wall is the main route by which a ZnO film is formed. The mechanism calculated by quantum chemistry was applied in computational fluid dynamics (CFD) simulations using Fluent14.0 software, and the concentration distribution and gas reaction path of the reaction chamber were calculated and analyzed. Finally, a 9 gas phase reaction model and an 8 surface reaction model were established. Together with the transport model, a complete chemical reaction-transport reaction model was constructed for the ZnO-MOCVD cavity. The validity of the model was verified, and the optimum temperature range of DEZn and oxygen-stabilized growth of ZnO films was determined to be 673-873 K. Using the results of the chemical reaction transport model, the geometry and operation parameters of the reactor can be optimized to improve the characteristics of the epitaxial layer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Jin B. J. Bae S. H. Lee S. Y. Im S. Mater. Sci. Eng., B. 2000;71:301–305. doi: 10.1016/S0921-5107(99)00395-5. - DOI
-
- Chen Y. J. Shih Y. Y. Ho C. H. Du J. H. Fu Y. P. Ceram. Int. 2010;36:69–73. doi: 10.1016/j.ceramint.2009.06.018. - DOI
-
- Lim J. Shin K. Woo Kim H. Lee C. J. Lumin. 2004;109:181–185. doi: 10.1016/S0022-2313(04)00142-5. - DOI
-
- Tsukazaki A. Ohtomo A. Onuma T. Ohtani M. Makino T. Sumiya M. Ohtani K. Chichibu S. F. Fuke S. Segawa Y. Nat. Mater. 2010;4:42–46. doi: 10.1038/nmat1284. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

