A small molecule targeting glutathione activates Nrf2 and inhibits cancer cell growth through promoting Keap-1 S-glutathionylation and inducing apoptosis
- PMID: 35538996
- PMCID: PMC9076930
- DOI: 10.1039/c7ra11935f
A small molecule targeting glutathione activates Nrf2 and inhibits cancer cell growth through promoting Keap-1 S-glutathionylation and inducing apoptosis
Abstract
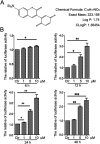
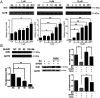
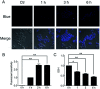
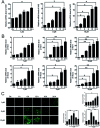
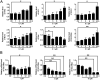
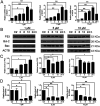
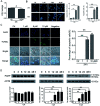
The level of glutathione (GSH) is increased in many cancer cells. Consuming intracellular GSH by chemical small molecules that specifically target GSH is a new strategy to treat cancer. Recently, we synthesized and proved that a new compound 2-(7-(diethylamino)-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione (PBQC) could target to and consume intracellular GSH specifically, but, it is not clear if PBQC can affect cancer cell growth and the activity of the nuclear factor-erythroid 2-related factor 2 (Nrf2) which is a key factor involved in regulation of cancer cell growth. In this study, we addressed these questions. We found that PBQC suppressed cancer cell growth through increasing the activity of Nrf2, while it did not inhibit normal vascular endothelial cell growth. Furthermore, we demonstrated that PBQC can cause Keap-1 protein S-glutathionylation and promote Nrf2 nuclear translocation as well as the expression of pro-apoptosis genes. As a result, the cancer cells underwent apoptosis. Here, we provide a new Nrf2 activator, PBQC that can promote the expressions of pro-apoptosis genes downstream Nrf2. The data suggest that PBQC is a potential lead-compound for development of new anti-cancer drugs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Epalrestat increases glutathione, thioredoxin, and heme oxygenase-1 by stimulating Nrf2 pathway in endothelial cells.Redox Biol. 2015;4:87-96. doi: 10.1016/j.redox.2014.12.002. Epub 2014 Dec 10. Redox Biol. 2015. PMID: 25529839 Free PMC article.
-
Combination of cyanidin-3-O-glucoside and cisplatin induces oxidative stress and apoptosis in HeLa cells by reducing activity of endogenous antioxidants, increasing bax/bcl-2 mRNA expression ratio, and downregulating Nrf2 expression.J Food Biochem. 2021 Jul;45(7):e13806. doi: 10.1111/jfbc.13806. Epub 2021 Jun 2. J Food Biochem. 2021. PMID: 34080212
-
Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation.Am J Pathol. 2008 Jun;172(6):1529-41. doi: 10.2353/ajpath.2008.070429. Epub 2008 May 5. Am J Pathol. 2008. PMID: 18458092 Free PMC article.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
[Recent advances in the study of Nrf2 and inflammatory respiratory diseases].Yao Xue Xue Bao. 2015 Sep;50(9):1080-7. Yao Xue Xue Bao. 2015. PMID: 26757542 Review. Chinese.
Cited by
-
Post-translational modifications of Keap1: the state of the art.Front Cell Dev Biol. 2024 Jan 8;11:1332049. doi: 10.3389/fcell.2023.1332049. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38259518 Free PMC article. Review.
-
Regulation of Cell Proliferation and Nrf2-Mediated Antioxidant Defense: Conservation of Keap1 Cysteines and Nrf2 Binding Site in the Context of the Evolution of KLHL Family.Life (Basel). 2023 Apr 19;13(4):1045. doi: 10.3390/life13041045. Life (Basel). 2023. PMID: 37109574 Free PMC article.
-
Beyond repression of Nrf2: An update on Keap1.Free Radic Biol Med. 2020 Sep;157:63-74. doi: 10.1016/j.freeradbiomed.2020.03.023. Epub 2020 Mar 28. Free Radic Biol Med. 2020. PMID: 32234331 Free PMC article. Review.
-
Riboflavin improves meat quality, antioxidant capacity, muscle development, and lipids composition of breast muscle in pigeon.Poult Sci. 2025 Mar;104(3):104856. doi: 10.1016/j.psj.2025.104856. Epub 2025 Jan 25. Poult Sci. 2025. PMID: 39970516 Free PMC article.
-
3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade.Molecules. 2021 Nov 8;26(21):6755. doi: 10.3390/molecules26216755. Molecules. 2021. PMID: 34771164 Free PMC article. Review.
References
-
- Suess E. Malessa S. Ungersbock K. Kitz P. Podreka I. Heimberger K. Hornykiewicz O. Deecke L. J. Nucl. Med. 1991;32:1675–1681. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

