Recent advances in metallopolymer-based drug delivery systems
- PMID: 35539076
- PMCID: PMC9075603
- DOI: 10.1039/c9ra06678k
Recent advances in metallopolymer-based drug delivery systems
Abstract
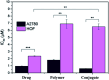
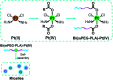
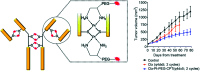
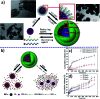
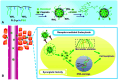
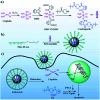
Metallopolymers (MPs) or metal-containing polymers have shown great potential as new drug delivery systems (DDSs) due to their unique properties, including universal architectures, composition, properties and surface chemistry. Over the past few decades, the exponential growth of many new classes of MPs that deal with these issues has been demonstrated. This review presents and assesses the recent advances and challenges associated with using MPs as DDSs. Among the most widely used MPs for these purposes, metal complexes based on synthetic and natural polymers, coordination polymers, metal-organic frameworks, and metallodendrimers are distinguished. Particular attention is paid to the stimulus- and multistimuli-responsive metallopolymer-based DDSs. Of considerable interest is the use of MPs for combination therapy and multimodal systems. Finally, the problems and future prospects of using metallopolymer-based DDSs are outlined. The bibliography includes articles published over the past five years.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures












































Similar articles
-
Applications of Metal-Organic Frameworks as Drug Delivery Systems.Int J Mol Sci. 2022 Apr 18;23(8):4458. doi: 10.3390/ijms23084458. Int J Mol Sci. 2022. PMID: 35457275 Free PMC article. Review.
-
Self-healing and shape memory metallopolymers: state-of-the-art and future perspectives.Dalton Trans. 2020 Mar 14;49(10):3042-3087. doi: 10.1039/c9dt04360h. Epub 2020 Feb 12. Dalton Trans. 2020. PMID: 32048663 Review.
-
Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review.Anticancer Agents Med Chem. 2021;21(18):2487-2504. doi: 10.2174/1871520621666210119093844. Anticancer Agents Med Chem. 2021. PMID: 33463479 Review.
-
Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications.Curr Pharm Des. 2019;25(11):1172-1186. doi: 10.2174/1381612825666190425190754. Curr Pharm Des. 2019. PMID: 31465278 Review.
-
Recent Trends in Metallopolymer Design: Redox-Controlled Surfaces, Porous Membranes, and Switchable Optical Materials Using Ferrocene-Containing Polymers.Chemistry. 2018 Jul 17;24(40):10006-10021. doi: 10.1002/chem.201800412. Epub 2018 May 4. Chemistry. 2018. PMID: 29532972 Review.
Cited by
-
Copper-Chelated Hyperbranched Polyethyleneimines with Antifungal Activity against quiescent conidia and germlings of the opportunistic fungal pathogen Aspergillus nidulans.Res Sq [Preprint]. 2025 May 30:rs.3.rs-5895555. doi: 10.21203/rs.3.rs-5895555/v1. Res Sq. 2025. Update in: Sci Rep. 2025 Jul 16;15(1):25711. doi: 10.1038/s41598-025-11018-6. PMID: 40502771 Free PMC article. Updated. Preprint.
-
Applications of Metal-Organic Frameworks as Drug Delivery Systems.Int J Mol Sci. 2022 Apr 18;23(8):4458. doi: 10.3390/ijms23084458. Int J Mol Sci. 2022. PMID: 35457275 Free PMC article. Review.
-
Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications.Molecules. 2024 May 15;29(10):2321. doi: 10.3390/molecules29102321. Molecules. 2024. PMID: 38792182 Free PMC article. Review.
-
The effect of copper on the antifungal activity of polyethyleneimines against quiescent conidia and germlings of Aspergillus nidulans.Sci Rep. 2025 Jul 16;15(1):25711. doi: 10.1038/s41598-025-11018-6. Sci Rep. 2025. PMID: 40670533 Free PMC article.
-
Development and evaluation of a Cu(II) complex as nanosuspension for enhanced antitumor efficacy against glioblastoma cancer.Sci Rep. 2025 Jul 26;15(1):27304. doi: 10.1038/s41598-025-13081-5. Sci Rep. 2025. PMID: 40715522 Free PMC article.
References
-
- Kim Y.-C. Min K. A. Jang D.-J. Ahn T. Y. Min J. H. Yu B. E. Cho K. H. J. Pharm. Invest. doi: 10.1007/s40005-019-00452-0. - DOI
-
- Vadlapudi A. D., Kwatra D. and Mitra A. K., in Drug Delivery, ed. A. K. Mitra, D. Kwatra and A. D. Vadlapudi, Jones & Bartlett Learning, Burlington, USA, 2015, pp. 1–7
-
- Duarte Y. Márquez-Miranda V. Miossec M. J. González-Nilo F. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2019;11:e1554. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

