Antiacetylcholinesterase triterpenes from the fruits of Cimicifuga yunnanensis
- PMID: 35539105
- PMCID: PMC9078504
- DOI: 10.1039/c8ra00291f
Antiacetylcholinesterase triterpenes from the fruits of Cimicifuga yunnanensis
Abstract
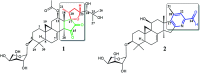
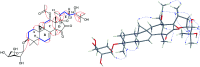
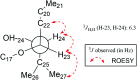
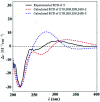
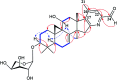
Two new cycloartane triterpenes, cimyunnin E (1), containing a unique oxaspiro[4.4]nonanedione moiety based on rings D and E, together with cimicifine B (2), a 25,26,27-trinortriterpene featuring a pyridine ring E, were purified from the fruits of Cimicifuga yunnanensis. Their structures were elucidated by spectroscopic methods and ECD (electronic circular dichroism calculations). Compounds 1 and 2 showed significant acetylcholinesterase (AChE) inhibition with IC50 values of 1.58 and 3.87 μM, respectively. In addition, they noticeably enhanced the neurite outgrowth of nerve growth factor (NGF) mediated PC12 cells at a concentration of 10 μM.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Similar articles
-
Cytotoxic 9,19-cycloartane triterpenes from the aerial parts of Cimicifuga yunnanensis.Fitoterapia. 2014 Dec;99:191-7. doi: 10.1016/j.fitote.2014.05.019. Epub 2014 Jun 2. Fitoterapia. 2014. PMID: 24887699
-
Cycloartane triterpene glycosides from rhizomes of Cimicifuga foetida L. with lipid-lowering activity on 3T3-L1 adipocytes.Fitoterapia. 2020 Sep;145:104635. doi: 10.1016/j.fitote.2020.104635. Epub 2020 May 26. Fitoterapia. 2020. PMID: 32464254
-
New anti-angiogenic leading structure discovered in the fruit of Cimicifuga yunnanensis.Sci Rep. 2015 Mar 12;5:9026. doi: 10.1038/srep09026. Sci Rep. 2015. PMID: 25762443 Free PMC article.
-
New triterpenes from Cimicifuga yunnanensis down-regulating the mRNA expression of CD147, MMP-2, and MMP-9.RSC Adv. 2021 Nov 17;11(58):36978-36988. doi: 10.1039/d1ra07828c. eCollection 2021 Nov 10. RSC Adv. 2021. PMID: 35494395 Free PMC article.
-
Cyathane Diterpenes from Cultures of the Bird's Nest Fungus Cyathus hookeri and Their Neurotrophic and Anti-neuroinflammatory Activities.J Nat Prod. 2019 Jun 28;82(6):1599-1608. doi: 10.1021/acs.jnatprod.9b00091. Epub 2019 Jun 18. J Nat Prod. 2019. PMID: 31244147 Review.
Cited by
-
Neurotrophic Natural Products.Prog Chem Org Nat Prod. 2024;123:1-473. doi: 10.1007/978-3-031-42422-9_1. Prog Chem Org Nat Prod. 2024. PMID: 38340248
-
Discovery and synthesis of novel beesioside I derivatives with potent anti-HIV activity.Eur J Med Chem. 2019 Mar 15;166:159-166. doi: 10.1016/j.ejmech.2019.01.020. Epub 2019 Jan 10. Eur J Med Chem. 2019. PMID: 30703659 Free PMC article.
References
-
- Liske E. Wustenberg P. Menopause. 1998;5:250–255. doi: 10.1097/00042192-199805040-00051. - DOI
-
- McKenna D. J. Jones K. Humphrey S. Hughes K. Altern. Ther. 2001;7:93–100. - PubMed
-
- Chinese Pharmacopoeia Commission, The Pharmacopoeia of Chinese People's Republic, ed. Y. Li, The Chemical Industry Publishing House, Beijing, China, 2010, vol. 1, pp. 68–69
LinkOut - more resources
Full Text Sources

