A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts
- PMID: 35539148
- PMCID: PMC9078493
- DOI: 10.1039/c7ra13546g
A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts
Abstract
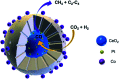
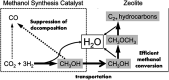
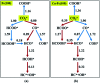
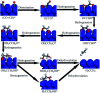
CO2 hydrogenation to hydrocarbons is a promising way of making waste to wealth and energy storage, which also solves the environmental and energy issues caused by CO2 emissions. Much efforts and research are aimed at the conversion of CO2 via hydrogenation to various value-added hydrocarbons, such as CH4, lower olefins, gasoline, or long-chain hydrocarbons catalyzed by different catalysts with various mechanisms. This review provides an overview of advances in CO2 hydrogenation to hydrocarbons that have been achieved recently in terms of catalyst design, catalytic performance and reaction mechanism from both experiments and density functional theory calculations. In addition, the factors influencing the performance of catalysts and the first C-C coupling mechanism through different routes are also revealed. The fundamental factor for product selectivity is the surface H/C ratio adjusted by active metals, supports and promoters. Furthermore, the technical and application challenges of CO2 conversion into useful fuels/chemicals are also summarized. To meet these challenges, future research directions are proposed in this review.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



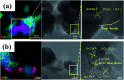

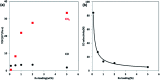
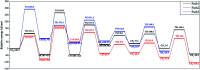
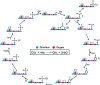

References
-
- Kahn B., Earth's CO2 Passes the 400 PPM Threshold—Maybe Permanently, Climate Central, 2016
-
- A Roadmap for moving to a competitive low carbon economy in 2050, European Commission, 2011
-
- Dimitriou I. García-Gutiérrez P. Elder R. H. Cuéllar-Franca R. M. Azapagic A. Allen R. W. K. Energy Environ. Sci. 2015;8:1775–1789.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

