Pyrrolidine and oxazolidine ring transformations in proline and serine derivatives of α-hydroxyphosphonates induced by deoxyfluorinating reagents
- PMID: 35539185
- PMCID: PMC9082089
- DOI: 10.1039/c8ra05186k
Pyrrolidine and oxazolidine ring transformations in proline and serine derivatives of α-hydroxyphosphonates induced by deoxyfluorinating reagents
Abstract
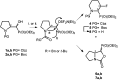
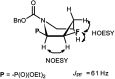
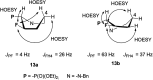
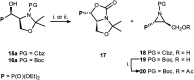
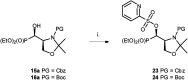
Transformations of α-hydroxyphosphonates derived from proline or serine by treatment with different deoxyfluorinating reagents (DAST, Deoxofluor, PyFluor) are reported. Depending on the applied reagent, as well as the protecting group used (N-Cbz, N-Boc, N-Bn) different types of products are observed. The reaction of N-Cbz or N-Boc prolinols with DAST or Deoxofluor due to aziridinium intermediate participation gave fluorinated amino phosphonates such as piperidine and pyrrolidine derivatives and/or oxazolidine-2-ones. Similarly, the analogous reaction of N-Cbz or N-Boc protected serinol yielded oxazolidine-2-ones or its fluorinated analogues. As the second type of product formed by DAST-induced reaction of serine derivatives, aziridines were obtained. Only in the case of deoxyfluorination of N-benzyl prolinols were both diastereoisomers of β-fluoropiperidine-α-phosphonates formed, while the reaction of protected N-benzyl serinols gave fluorinated oxazolidines. Moreover, application of PyFluor gave sulfonate derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
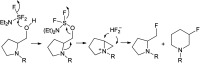



Similar articles
-
Deoxyfluorinating reagents as tools for γ-amino-α-hydroxyphosphonate modification.Org Biomol Chem. 2022 Jul 20;20(28):5615-5623. doi: 10.1039/d2ob00915c. Org Biomol Chem. 2022. PMID: 35796647
-
Functionalization of α-hydroxyphosphonates as a convenient route to N-tosyl-α-aminophosphonates.RSC Adv. 2018 Mar 27;8(22):11957-11974. doi: 10.1039/c8ra01656a. eCollection 2018 Mar 26. RSC Adv. 2018. PMID: 35539392 Free PMC article.
-
Design and Use of an Oxazolidine Silyl Enol Ether as a New Homoalanine Carbanion Equivalent for the Synthesis of Carbon-Linked Isosteres of O-Glycosyl Serine and N-Glycosyl Asparagine.J Org Chem. 1999 Feb 5;64(3):933-944. doi: 10.1021/jo981861h. J Org Chem. 1999. PMID: 11674165
-
[Synthesis of phosphonic acid and phosphinic acid derivatives for development of biologically active compounds].Yakugaku Zasshi. 2004 Nov;124(11):725-49. doi: 10.1248/yakushi.124.725. Yakugaku Zasshi. 2004. PMID: 15516802 Review. Japanese.
-
Synthesis, structure, and biological applications of α-fluorinated β-amino acids and derivatives.Chem Biodivers. 2012 Nov;9(11):2410-41. doi: 10.1002/cbdv.201200307. Chem Biodivers. 2012. PMID: 23161626 Review.
Cited by
-
Access to 2-Fluorinated Aziridine-2-phosphonates from α,α-Halofluorinated β-Iminophosphonates-Spectroscopic and Theoretical Studies.Molecules. 2023 Jul 22;28(14):5579. doi: 10.3390/molecules28145579. Molecules. 2023. PMID: 37513451 Free PMC article.
-
Reactions of Piperazin-2-one, Morpholin-3-one, and Thiomorpholin-3-one with Triethyl Phosphite Prompted by Phosphoryl Chloride: Scope and Limitations.ACS Omega. 2019 May 22;4(5):9056-9064. doi: 10.1021/acsomega.9b01137. eCollection 2019 May 31. ACS Omega. 2019. PMID: 31459993 Free PMC article.
-
Highly Diastereoselective Construction of Carbon- Heteroatom Quaternary Stereogenic Centers in the Synthesis of Analogs of Bioactive Compounds: From Monofluorinated Epoxyalkylphosphonates to α-Fluoro-, β-, or γ-Amino Alcohol Derivatives of Alkylphosphonates.Front Chem. 2021 Jun 2;9:613633. doi: 10.3389/fchem.2021.613633. eCollection 2021. Front Chem. 2021. PMID: 34150715 Free PMC article.
References
-
- Aminophosphonic and Aminophosphinic Acids, ed. V. P. Kukhar and H. R. Hudson, John Wiley & Sons Ltd, Chichester, 2000; For some review see
- Kafarski P. Lejczak B. Curr. Med. Chem.: Anti-Cancer Agents. 2001;1:301–312. doi: 10.2174/1568011013354543. - DOI - PubMed
- Grembecka J. Kafarski P. Mini-Rev. Med. Chem. 2001;1:133–144. doi: 10.2174/1389557013406990. - DOI - PubMed
- Ordóñez M. Rojas-Cabrera H. Cativiela C. Tetrahedron. 2009;65:17–49. doi: 10.1016/j.tet.2008.09.083. - DOI - PMC - PubMed
- Van der Jeught S. Stevens Ch. S. Chem. Rev. 2009;109:2672–2702. doi: 10.1021/cr800315j. - DOI - PubMed
- Orsini F. Sello G. Sisti M. Curr. Med. Chem. 2010;17:264–289. doi: 10.2174/092986710790149729. - DOI - PubMed
- Naydenova E. D. Todorov P. T. Troev K. D. Amino Acids. 2010;38:23–30. doi: 10.1007/s00726-009-0254-7. - DOI - PubMed
- Ordóñez M. Viveros-Ceballos J. L. Cativiela C. Sayago F. J. Tetrahedron. 2015;71:1745–1784. doi: 10.1016/j.tet.2015.01.029. - DOI
-
- Boduszek B. Oleksyszyn J. Kam C.-M. Selzler J. Smith R. E. Powers J. C. J. Med. Chem. 1994;37:3969–3976. doi: 10.1021/jm00049a016. - DOI - PubMed
- Lambeir A. M. Borloo M. De Meester I. Belyaev A. Augustyns K. Hendriks D. Scharpé S. Haemers A. Biochim. Biophys. Acta. 1996;1290:76–82. doi: 10.1016/0304-4165(96)00012-8. - DOI - PubMed
-
- Surh Y. Spencer R. P. Spitznagle L. A. Hosain F. Lejczak B. J. Nucl. Med. 1986;27:847–849. - PubMed
-
- Katritzky A. R. Cui X.-L. Yang B. Steel P. J. J. Org. Chem. 1999;64:1979–1985. doi: 10.1021/jo9821426. - DOI - PubMed
- Dinér P. Amedjkouh M. Org. Biomol. Chem. 2006;4:2091–2096. doi: 10.1039/B605091C. - DOI - PubMed
- Tao Q. Tang G. Lin K. Zhao Y.-F. Chirality. 2008;20:833–838. doi: 10.1002/chir.20552. - DOI - PubMed
- Hirata S. Kuriyama M. Onomura O. Tetrahedron. 2011;67:9411–9416. doi: 10.1016/j.tet.2011.09.080. - DOI
- Ma T. Fu X. Kee C. W. Zong L. Pan Y. Huang K.-W. Tan C.-H. J. Am. Chem. Soc. 2011;133:2828–2831. doi: 10.1021/ja1098353. - DOI - PubMed
- Wuggenig F. Schweifer A. Mereiter K. Hammerschmidt F. Eur. J. Org. Chem. 2011:1870–1879. doi: 10.1002/ejoc.201001501. - DOI
- Kaboudin B. Kato J.-Y. Aoyama H. Yokomatsu T. Tetrahedron: Asymmetry. 2013;24:1562–1566. doi: 10.1016/j.tetasy.2013.10.013. - DOI
- Kudzin M. H. Kudzin Z. H. Drabowicz J. ARKIVOC. 2011;vi:227–269.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

