Experimental and theoretical approaches for the selective detection of thymine in real samples using gold nanoparticles as a biochemical sensor
- PMID: 35539214
- PMCID: PMC9082146
- DOI: 10.1039/c8ra02627k
Experimental and theoretical approaches for the selective detection of thymine in real samples using gold nanoparticles as a biochemical sensor
Abstract
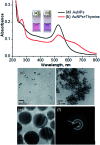
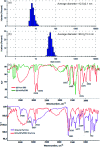
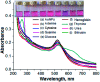
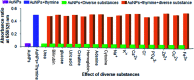
We report a simple, selective and cost effective method for the qualitative and quantitative determination of thymine in a DNA standard and urine samples using gold nanoparticles (AuNPs) as a label-free colorimetric biochemical sensor. The mechanism for the detection of thymine is demonstrated via the color change of the AuNPs from pink to blue, followed by the shift of the localized surface plasmon resonance (LSPR) absorption band to a higher wavelength with the introduction of an analyte. The selective detection of thymine was experimentally verified by performing a control experiment with nucleobases, other biomolecules, metal ions and anions. In addition, the computation density functional theory (DFT) and time dependent density functional theory (TD-DFT) using the Gaussian (C.01) program highlighted that the electrostatic potential behavior of the thymine molecule facilitated a non-covalent interaction toward gold for the selective detection of analytes, and the computation was also used to calculate a UV-Vis absorption band as well. The calculated absorption band of the AuNPs with thymine, obtained using TD-DFT, was found to be very close to the experimental data. The omnicapped truncated tetrahedral (ν 3-tetrahedral) Au20 cluster structure was considered as the model for the AuNP optimization. The linear range obtained for the quantitative determination of thymine was found to be 10-1200 ng mL-1 with a limit of detection of 3 ng mL-1. The advantages of using the AuNPs as a biochemical sensor are that they provide a facile and low cost method and are selective for the qualitative and quantitative determination of thymine in a DNA standard and in urine samples in comparison to chromatographic and electrochemical methods.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
A wavelength-modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury(II) ion by gold nanoparticles-DNA conjugates.Biosens Bioelectron. 2018 Aug 30;114:15-21. doi: 10.1016/j.bios.2018.05.004. Epub 2018 May 8. Biosens Bioelectron. 2018. PMID: 29775854
-
DNA functionalized gold nanorods/nanoplates assembly as sensitive LSPR-based sensor for label-free detection of mercury ions.Colloids Surf B Biointerfaces. 2013 Oct 1;110:485-8. doi: 10.1016/j.colsurfb.2013.04.039. Epub 2013 Apr 30. Colloids Surf B Biointerfaces. 2013. PMID: 23693125
-
Sucrose capped gold nanoparticles as a plasmonic chemical sensor based on non-covalent interactions: Application for selective detection of vitamins B1 and B6 in brown and white rice food samples.Food Chem. 2018 Jun 1;250:14-21. doi: 10.1016/j.foodchem.2018.01.002. Epub 2018 Jan 2. Food Chem. 2018. PMID: 29412903
-
Onsite-detection of barium and nickel from river, pond and tap water samples using gold nanoparticles as a chemical sensor.Spectrochim Acta A Mol Biomol Spectrosc. 2017 Feb 15;173:630-636. doi: 10.1016/j.saa.2016.10.020. Epub 2016 Oct 17. Spectrochim Acta A Mol Biomol Spectrosc. 2017. PMID: 27776318
-
Perspectives of characterization and bioconjugation of gold nanoparticles and their application in lateral flow immunosensing.Drug Deliv Transl Res. 2020 Aug;10(4):878-902. doi: 10.1007/s13346-020-00771-y. Drug Deliv Transl Res. 2020. PMID: 32367423 Review.
Cited by
-
A simple and cost-effective paper-based and colorimetric dual-mode detection of arsenic(iii) and lead(ii) based on glucose-functionalized gold nanoparticles.RSC Adv. 2021 Jun 10;11(34):20769-20780. doi: 10.1039/d1ra02929k. eCollection 2021 Jun 9. RSC Adv. 2021. PMID: 35479386 Free PMC article.
-
Au-Ag core-shell composite nanoparticles as a selective and sensitive plasmonic chemical probe for l-cysteine detection in Lens culinaris (lentils).RSC Adv. 2021 Jun 7;11(33):20380-20390. doi: 10.1039/d1ra01824h. eCollection 2021 Jun 3. RSC Adv. 2021. PMID: 35479888 Free PMC article.
-
Tackling breast cancer with gold nanoparticles: twinning synthesis and particle engineering with efficacy.Nanoscale Adv. 2024 Apr 17;6(11):2766-2812. doi: 10.1039/d3na00988b. eCollection 2024 May 29. Nanoscale Adv. 2024. PMID: 38817429 Free PMC article. Review.
References
-
- Berg J. M., Tymoczko J. L. and Stryer L., Biochemistry, WH Freeman and Company, 5th edn, 2002, pp. 118–119
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. and Walter P., Molecular Biology of the Cell, 6th edn, 2014, ch. 4, DNA, Chromosomes and Genomes, Garland Science, pp. 1–1464
-
- Sheng R. S. Ni F. Cotton T. M. J. Anal. Chem. 1991;63:437–442.
-
- Brohi R. O. Z. Z. Khuhawar M. Y. Khuhawar T. M. J. Anal. Sci. Technol. 2016;7:1–6. doi: 10.1186/s40543-015-0080-3. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

