A series of water-soluble photosensitizers based on 3-cinnamoylcoumarin for in vitro antimicrobial photodynamic inactivation
- PMID: 35539218
- PMCID: PMC9080500
- DOI: 10.1039/c8ra02557f
A series of water-soluble photosensitizers based on 3-cinnamoylcoumarin for in vitro antimicrobial photodynamic inactivation
Abstract
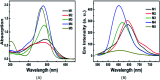
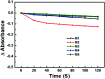
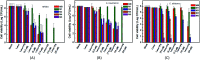
A series of novel water-soluble photosensitizers (PSs; M1-M5) based on 3-cinnamoylcoumarin derivatives, incorporating carboxylic acid salt (M1, M2), pyridine salt (M3, M4) and quaternary ammonium salt (M5) groups, were designed and synthesized. Their photophysical and photochemical properties and in vitro antimicrobial photodynamic inactivation (PDI) were investigated. M2, modified with two carboxylic acid salts, was unstable in phosphate-buffered saline (PBS). The four other PSs all showed higher binding/uptake to methicillin-resistant Staphylococcus aureus (MRSA), A. baumannii and C. albicans compared with the clinical drug methylene blue (MB), except for M1 to A. baumannii. Furthermore, the three cationic PSs (M3-M5) exhibited equivalent antibacterial PDI efficacies against MRSA and A. baumannii compared with MB. The antifungal efficacies of M4 and M5 to C. albicans were both significantly higher than that of MB, especially for M5, indicating that the quaternary ammonium-salt-modified coumarin derivative has substantial potential for antifungal PDI.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Cationic, anionic and neutral dyes: effects of photosensitizing properties and experimental conditions on the photodynamic inactivation of pathogenic bacteria.J Water Health. 2013 Dec;11(4):590-9. doi: 10.2166/wh.2013.219. J Water Health. 2013. PMID: 24334833
-
Photodynamic inactivation of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus with Ru(II)-based type I/type II photosensitizers.Photodiagnosis Photodyn Ther. 2013 Dec;10(4):615-25. doi: 10.1016/j.pdpdt.2013.07.001. Epub 2013 Aug 5. Photodiagnosis Photodyn Ther. 2013. PMID: 24284119
-
In vitro photodynamic inactivation effects of benzylidene cyclopentanone photosensitizers on clinical fluconazole-resistant Candida albicans.Photodiagnosis Photodyn Ther. 2018 Jun;22:178-186. doi: 10.1016/j.pdpdt.2018.04.001. Epub 2018 Apr 4. Photodiagnosis Photodyn Ther. 2018. PMID: 29626527
-
Contemporary approaches and future perspectives of antibacterial photodynamic therapy (aPDT) against methicillin-resistant Staphylococcus aureus (MRSA): A systematic review.Eur J Med Chem. 2020 Aug 15;200:112341. doi: 10.1016/j.ejmech.2020.112341. Epub 2020 May 13. Eur J Med Chem. 2020. PMID: 32505848
-
Ecophysiological steps of marine adaptation in extant and extinct non-avian tetrapods.Biol Rev Camb Philos Soc. 2021 Oct;96(5):1769-1798. doi: 10.1111/brv.12724. Epub 2021 Apr 26. Biol Rev Camb Philos Soc. 2021. PMID: 33904243 Review.
Cited by
-
Serendipitous Discovery of Photolytic Thiosulfoxide Formation: Application for Visible-Light-Inducible Manipulation of Supersulfide Level in Biological Systems.J Am Chem Soc. 2025 Apr 16;147(15):12627-12634. doi: 10.1021/jacs.5c00196. Epub 2025 Apr 1. J Am Chem Soc. 2025. PMID: 40169142 Free PMC article.
References
-
- Laxminarayan R. Duse A. Wattal C. Zaidi A. K. M. Wertheim H. F. L. Sumpradit N. Vlieghe E. Hara G. L. Gould I. M. Goossens H. Greko C. So A. D. Bigdeli M. Tomson G. Woodhouse W. Ombaka E. Peralta A. Q. Qamar F. N. Mir F. Kariuki S. Bhutta Z. A. Coates A. Bergstrom R. Wright G. D. Brown E. D. Cars O. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. - DOI - PubMed
-
- Ferraz R. Teixeira V. Rodrigues D. Fernandes R. Prudêncio C. Noronha J. P. Petrovski Ž. Branco L. C. RSC Adv. 2014;4:4301–4307. doi: 10.1039/C3RA44286A. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

