Design, synthesis and biological evaluation of N-arylsulfonyl carbazoles as novel anticancer agents
- PMID: 35539273
- PMCID: PMC9080423
- DOI: 10.1039/c8ra02939c
Design, synthesis and biological evaluation of N-arylsulfonyl carbazoles as novel anticancer agents
Abstract
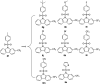
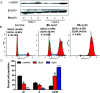
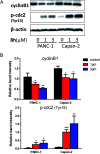
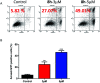
In this work, a set of structurally diverse synthetic carbazoles was screened for their anticancer activities. According to structure-activity relationship studies, carbazoles with an N-substituted sulfonyl group exhibited better anticancer activity. Moreover, compound 8h was discovered to show the most potent anticancer effects on Capan-2 cells by inducing apoptosis and cell cycle arrest in G2/M phase. Finally, the in vivo study demonstrated that 8h prevented the tumor growth in PANC-1 and Capan-2 xenograft models without apparent toxicity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Synthesis and biological evaluation of N(9)-substituted harmine derivatives as potential anticancer agents.Bioorg Med Chem Lett. 2016 Aug 15;26(16):4015-9. doi: 10.1016/j.bmcl.2016.06.087. Epub 2016 Jul 1. Bioorg Med Chem Lett. 2016. PMID: 27397495
-
Novel benzotriazole N-acylarylhydrazone hybrids: Design, synthesis, anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis and FAK inhibition.Bioorg Chem. 2018 Oct;80:531-544. doi: 10.1016/j.bioorg.2018.07.008. Epub 2018 Jul 10. Bioorg Chem. 2018. PMID: 30014921
-
Design and synthesis of 6,7-methylenedioxy-4-substituted phenylquinolin-2(1H)-one derivatives as novel anticancer agents that induce apoptosis with cell cycle arrest at G2/M phase.Bioorg Med Chem. 2013 Sep 1;21(17):5064-75. doi: 10.1016/j.bmc.2013.06.046. Epub 2013 Jun 26. Bioorg Med Chem. 2013. PMID: 23867385 Free PMC article.
-
Design, synthesis, and biological evaluation of novel benzodiazepine derivatives as anticancer agents through inhibition of tubulin polymerization in vitro and in vivo.Eur J Med Chem. 2019 Nov 15;182:111670. doi: 10.1016/j.ejmech.2019.111670. Epub 2019 Sep 2. Eur J Med Chem. 2019. PMID: 31499359
-
Design, Synthesis, biological Evaluation, and molecular docking studies of novel Pyrazolo[3,4-d]Pyrimidine derivative scaffolds as potent EGFR inhibitors and cell apoptosis inducers.Bioorg Chem. 2021 Nov;116:105325. doi: 10.1016/j.bioorg.2021.105325. Epub 2021 Sep 4. Bioorg Chem. 2021. PMID: 34507234
Cited by
-
Anti-Breast Cancer Properties and In Vivo Safety Profile of a Bis-Carbazole Derivative.Pharmaceutics. 2025 Mar 25;17(4):415. doi: 10.3390/pharmaceutics17040415. Pharmaceutics. 2025. PMID: 40284411 Free PMC article.
-
Synthesis of N-acyl carbazoles, phenoxazines and acridines from cyclic diaryliodonium salts.Beilstein J Org Chem. 2024 Jan 4;20:12-16. doi: 10.3762/bjoc.20.2. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38213840 Free PMC article.
-
Synthesis and Antiproliferative Activity of Novel Dehydroabietic Acid-Chalcone Hybrids.Molecules. 2022 Jun 5;27(11):3623. doi: 10.3390/molecules27113623. Molecules. 2022. PMID: 35684559 Free PMC article.
-
Coumarin-carbazole based functionalized pyrazolines: synthesis, characterization, anticancer investigation and molecular docking.RSC Adv. 2021 Aug 13;11(44):27627-27644. doi: 10.1039/d1ra03970a. eCollection 2021 Aug 9. RSC Adv. 2021. PMID: 35480680 Free PMC article.
References
-
- Pieper A. A. Xie S. Capota E. Estill S. J. Zhong J. Long J. M. Becker G. L. Huntington P. Goldman S. E. Shen C. H. Capota M. Britt J. K. Kotti T. Ure K. Brat D. J. Williams N. S. MacMillan K. S. Naidoo J. Melito L. Hsieh J. De Brabander J. Ready J. M. McKnight S. L. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

