1,3,5-Triphenylbenzene: a versatile photoluminescent chemo-sensor platform and supramolecular building block
- PMID: 35539277
- PMCID: PMC9081830
- DOI: 10.1039/c8ra02658k
1,3,5-Triphenylbenzene: a versatile photoluminescent chemo-sensor platform and supramolecular building block
Abstract
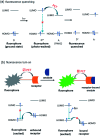
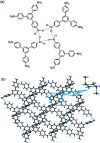
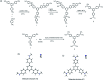
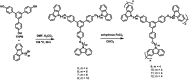
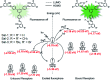
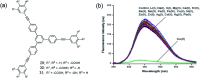
Fluorescence chemo-sensors for species of environmental and biological significance have emerged as a major research area in recent years. In this account, we describe fluorescence quenching as well as enhancement-based chemo-sensors obtained by employing C 3-symmetric 1,3,5-triphenylbenzene (1,3,5-TPB) as the fluorescence signalling unit. 1,3,5-TPB is a thermally and photochemically stable fluorescent platform with π-electron-rich characteristics. Starting from this platform, supramolecular, discrete, triphenylbenzene-carbazole, covalent-organic framework, covalent-organic polymer and conjugated polymer based sensors have been developed for the selective detection of polynitroaromatic compounds, trinitrotoluene (TNT), dinitrotoluene (DNT) and picric acid (PA). Tris-salicylaldimine Schiff bases have been synthesized for the selective sensing of fluoride ions through a fluorescence turn-on mechanism. It is likely that it should be possible to develop other highly selective and sensitive chemo-sensors by incorporating 1,3,5-TPB as the fluorophore unit.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures









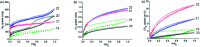



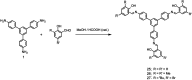





Similar articles
-
Triphenylbenzene Sensor for Selective Detection of Picric Acid.J Fluoresc. 2017 Jul;27(4):1299-1305. doi: 10.1007/s10895-017-2063-9. Epub 2017 Mar 14. J Fluoresc. 2017. PMID: 28293816
-
Three-Fold C 3-Symmetric Off-On Fluorescent Chemo-Sensors for Fluoride.J Fluoresc. 2016 May;26(3):997-1005. doi: 10.1007/s10895-016-1787-2. Epub 2016 Apr 11. J Fluoresc. 2016. PMID: 27067207
-
Selective fluorescence sensing of polynitroaromatic explosives using triaminophenylbenzene scaffolds.Phys Chem Chem Phys. 2014 Jun 14;16(22):10651-8. doi: 10.1039/c4cp00930d. Phys Chem Chem Phys. 2014. PMID: 24752547
-
π-Electron rich small molecule sensors for the recognition of nitroaromatics.Chem Commun (Camb). 2015 Nov 18;51(89):16014-32. doi: 10.1039/c5cc07513k. Chem Commun (Camb). 2015. PMID: 26463400
-
Self-assembled discrete molecules for sensing nitroaromatics.Chemistry. 2015 Apr 27;21(18):6656-66. doi: 10.1002/chem.201406092. Epub 2015 Feb 18. Chemistry. 2015. PMID: 25694365
Cited by
-
Blue Emitting Star-Shaped and Octasilsesquioxane-Based Polyanions Bearing Boron Clusters. Photophysical and Thermal Properties.Molecules. 2020 Mar 7;25(5):1210. doi: 10.3390/molecules25051210. Molecules. 2020. PMID: 32156041 Free PMC article.
-
β-Ketoenamine Covalent Organic Frameworks-Effects of Functionalization on Pollutant Adsorption.Polymers (Basel). 2022 Jul 29;14(15):3096. doi: 10.3390/polym14153096. Polymers (Basel). 2022. PMID: 35956612 Free PMC article.
-
Covalent Organic Frameworks: Synthesis, Properties and Applications-An Overview.Polymers (Basel). 2021 Mar 22;13(6):970. doi: 10.3390/polym13060970. Polymers (Basel). 2021. PMID: 33809960 Free PMC article. Review.
-
Click Chemistry Derived Hexa-ferrocenylated 1,3,5-Triphenylbenzene for the Detection of Divalent Transition Metal Cations.ACS Omega. 2024 Sep 5;9(37):38658-38667. doi: 10.1021/acsomega.4c04300. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310204 Free PMC article.
References
-
- Ma X. Tao F. Zhang Y. Li T. Raymo F. M. Cui Y. J. Mater. Chem. A. 2017;5:14343–14354.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

