Design, synthesis, and biological evaluation of AV6 derivatives as novel dual reactivators of latent HIV-1
- PMID: 35539279
- PMCID: PMC9080425
- DOI: 10.1039/c8ra01216d
Design, synthesis, and biological evaluation of AV6 derivatives as novel dual reactivators of latent HIV-1
Abstract
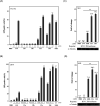
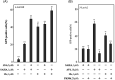
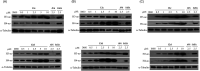
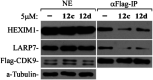
The "shock and kill" strategy might be a promising therapeutic approach for HIV/AIDS due to the existence of latent viral reservoirs. A major challenge of the "shock and kill" strategy arises from the general lack of clinically effective latency-reversing agents (LRAs). The 2-methylquinoline derivative, antiviral 6 (AV6) has been reported to induce latent HIV-1 expression and act synergistically with a HDAC inhibitor VA to reverse HIV latency. We report herein the design and identification of AV6 analogues which possess the zinc-binding group of HDAC inhibitors and have dual acting mechanism for the reactivation of HIV-1 from latency. Evaluation of compounds for the reactivation of HIV-1 latency identified two excellent active compounds 12c and 12d. Further bioassays revealed that these two compounds reactivated latent HIV-1 through dual mechanism, the inhibition of HDACs and NFAT-required for early HIV-1 gene expression. Additionally, it was found that 12c and 12d could reactivate HIV-1 transcription by releasing P-TEFb from the inactive complex 7SK snRNP. At last, molecular docking identified their orientation and binding interactions at the active site of HDAC2. This experimental data suggests that 12c and 12d can be served as effective HIV-1 LRAs which can be taken up for further studies.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
A chalcone derivative reactivates latent HIV-1 transcription through activating P-TEFb and promoting Tat-SEC interaction on viral promoter.Sci Rep. 2017 Sep 6;7(1):10657. doi: 10.1038/s41598-017-10728-w. Sci Rep. 2017. PMID: 28878233 Free PMC article.
-
Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus.J Virol. 2019 Aug 28;93(18):e00495-19. doi: 10.1128/JVI.00495-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243131 Free PMC article.
-
CPI-637 as a Potential Bifunctional Latency-Reversing Agent That Targets Both the BRD4 and TIP60 Proteins.Front Cell Infect Microbiol. 2021 Jul 19;11:686035. doi: 10.3389/fcimb.2021.686035. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34350133 Free PMC article.
-
Shocking HIV-1 with immunomodulatory latency reversing agents.Semin Immunol. 2021 Jan;51:101478. doi: 10.1016/j.smim.2021.101478. Epub 2021 May 8. Semin Immunol. 2021. PMID: 33972164 Review.
-
Current Status of Latency Reversing Agents Facing the Heterogeneity of HIV-1 Cellular and Tissue Reservoirs.Front Microbiol. 2020 Jan 24;10:3060. doi: 10.3389/fmicb.2019.03060. eCollection 2019. Front Microbiol. 2020. PMID: 32038533 Free PMC article. Review.
Cited by
-
Diversity of small molecule HIV-1 latency reversing agents identified in low- and high-throughput small molecule screens.Med Res Rev. 2020 May;40(3):881-908. doi: 10.1002/med.21638. Epub 2019 Oct 13. Med Res Rev. 2020. PMID: 31608481 Free PMC article. Review.
-
Medicinal Chemistry of Anti-HIV-1 Latency Chemotherapeutics: Biotargets, Binding Modes and Structure-Activity Relationship Investigation.Molecules. 2022 Dec 20;28(1):3. doi: 10.3390/molecules28010003. Molecules. 2022. PMID: 36615199 Free PMC article. Review.
References
-
- A. S. S. W. G. o. H. I. V. C. International Deeks S. G. Autran B. Berkhout B. Benkirane M. Cairns S. Chomont N. Chun T. W. Churchill M. Di Mascio M. Katlama C. Lafeuillade A. Landay A. Lederman M. Lewin S. R. Maldarelli F. Margolis D. Markowitz M. Martinez-Picado J. Mullins J. I. Mellors J. Moreno S. O'Doherty U. Palmer S. Penicaud M. C. Peterlin M. Poli G. Routy J. P. Rouzioux C. Silvestri G. Stevenson M. Telenti A. Van Lint C. Verdin E. Woolfrey A. Zaia J. Barre-Sinoussi F. Nat. Rev. Immunol. 2012;12:607–614. doi: 10.1038/nri3262. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

