Amine-functionalized MIL-53(Al) with embedded ruthenium nanoparticles as a highly efficient catalyst for the hydrolytic dehydrogenation of ammonia borane
- PMID: 35539406
- PMCID: PMC9079245
- DOI: 10.1039/c8ra01507d
Amine-functionalized MIL-53(Al) with embedded ruthenium nanoparticles as a highly efficient catalyst for the hydrolytic dehydrogenation of ammonia borane
Abstract
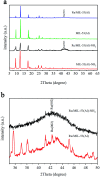
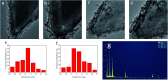
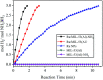
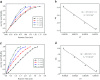
Well-dispersed ruthenium nanoparticles (Ru NPs) are immobilized within the pores of amine-functionalized MIL-53 via an in situ impregnation-reduction method. The resulting Ru/MIL-53(Al)-NH2 catalyst exhibits superior catalytic performance for the dehydrogenation of ammonia borane (AB) at ambient temperature relative to the Ru/MIL-53(Al) catalyst; it has a turnover frequency (TOF) of 287 mol H2 min-1 (mol Ru)-1 and an activation energy (E a) of 30.5 kJ mol-1. The amine groups present in the MIL-53(Al)-NH2 framework facilitate the formation and stabilization of ultra-small Ru NPs by preventing their aggregation. Additionally, the Ru/MIL-53(Al)-NH2 catalyst exhibits satisfactory durability and reusability: 72.4% and 86.3% of the initial catalytic activity was maintained after the fifth successive cycle of the hydrolytic dehydrogenation of AB in the two respective tests.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Nanozirconia supported ruthenium(0) nanoparticles: Highly active and reusable catalyst in hydrolytic dehydrogenation of ammonia borane.J Colloid Interface Sci. 2018 Mar 1;513:287-294. doi: 10.1016/j.jcis.2017.11.037. Epub 2017 Nov 13. J Colloid Interface Sci. 2018. PMID: 29156236
-
Ruthenium nanoparticles confined in SBA-15 as highly efficient catalyst for hydrolytic dehydrogenation of ammonia borane and hydrazine borane.Sci Rep. 2015 Oct 16;5:15186. doi: 10.1038/srep15186. Sci Rep. 2015. PMID: 26471355 Free PMC article.
-
Reducible tungsten(VI) oxide-supported ruthenium(0) nanoparticles: highly active catalyst for hydrolytic dehydrogenation of ammonia borane.Turk J Chem. 2023 Sep 28;47(5):1224-1238. doi: 10.55730/1300-0527.3607. eCollection 2023. Turk J Chem. 2023. PMID: 38173757 Free PMC article.
-
Hydrolytic dehydrogenation of NH3BH3 catalyzed by ruthenium nanoparticles supported on magnesium-aluminum layered double-hydroxides.RSC Adv. 2020 Mar 9;10(17):9996-10005. doi: 10.1039/d0ra01720e. eCollection 2020 Mar 6. RSC Adv. 2020. PMID: 35498595 Free PMC article.
-
The Hydrogen-Storage Challenge: Nanoparticles for Metal-Catalyzed Ammonia Borane Dehydrogenation.Small. 2021 Nov;17(44):e2102759. doi: 10.1002/smll.202102759. Epub 2021 Aug 19. Small. 2021. PMID: 34411437 Review.
Cited by
-
Synergetic Effect of Ultrasmall Metal Clusters and Zeolites Promoting Hydrogen Generation.Adv Sci (Weinh). 2019 Mar 25;6(10):1802350. doi: 10.1002/advs.201802350. eCollection 2019 May 17. Adv Sci (Weinh). 2019. PMID: 31131197 Free PMC article.
-
Anodic electrosynthesis of MIL-53(Al)-N(CH2PO3H2)2 as a mesoporous catalyst for synthesis of novel (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines via a cooperative vinylogous anomeric based oxidation.Sci Rep. 2021 Sep 29;11(1):19370. doi: 10.1038/s41598-021-97801-7. Sci Rep. 2021. PMID: 34588471 Free PMC article.
References
-
- Jena P. J. Phys. Chem. Lett. 2011;2:206–211. doi: 10.1021/jz1015372. - DOI
-
- Roy B. Manna J. Sharma P. J. Alloys Compd. 2015;645:234–238. doi: 10.1016/j.jallcom.2015.01.046. - DOI
-
- Wang H. Zhou L. Han M. Tao Z. Cheng F. Chen J. J. Alloys Compd. 2015;651:382–388. doi: 10.1016/j.jallcom.2015.08.139. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

