Novel pathway to produce high molecular weight kraft lignin-acrylic acid polymers in acidic suspension systems
- PMID: 35539425
- PMCID: PMC9079287
- DOI: 10.1039/c7ra12971h
Novel pathway to produce high molecular weight kraft lignin-acrylic acid polymers in acidic suspension systems
Abstract
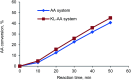
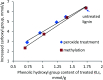
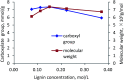
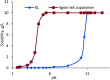
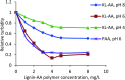
Kraft lignin (KL) produced in kraft pulping process has a low molecular weight and solubility, which limits its application in industry. For the first time, KL was polymerized with acrylic acid (AA) in an acidic aqueous suspension system to produce a water soluble lignin-AA polymer with a high molecular weight in this work. The polymerization reaction was carried out using K2S2O8 as an initiator, and the influence of reaction conditions on the carboxylate group content and molecular weight of resultant lignin polymers was systematically investigated. The mechanism of polymerization of KL and AA was discussed fundamentally. The resulting lignin-AA polymer was characterized by Fourier Transform Infrared spectrophotometry (FTIR), proton nuclear magnetic resonance (1H-NMR) and elemental analyses. The results showed that the phenolic hydroxyl group (Ph-OH) content of KL promoted the polymerization under an acidic environment. Under the conditions of 1.5 wt% of initiator, 3.5 of pH, 10.0 of AA/lignin molar ratio, 0.15 mol L-1 of lignin concentration, 3 h and 80 °C, the carboxylate group content and the molecular weight of the polymer were 7.37 mmol g-1 and 7.4 × 105 g mol-1, respectively. The lignin-AA polymer was water soluble at a 10 g L-1 concentration and a pH higher than 4.5. Furthermore, the flocculation performance of lignin-AA polymer in an aluminium oxide suspension was evaluated. Compared with polyAA, the lignin-AA polymer was a more efficient flocculant for aluminium oxide suspension, which shows its potential to be used as a green flocculant in industry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
Sulfonated Lignin-g-Styrene Polymer: Production and Characterization.Polymers (Basel). 2018 Aug 19;10(8):928. doi: 10.3390/polym10080928. Polymers (Basel). 2018. PMID: 30960853 Free PMC article.
-
Enhanced flocculation of aluminum oxide particles by lignin-based flocculants in dual polymer systems.J Environ Manage. 2023 Feb 15;328:116999. doi: 10.1016/j.jenvman.2022.116999. Epub 2022 Dec 13. J Environ Manage. 2023. PMID: 36516704
-
A new solvent-free pathway for inducing quaternized lignin-derived high molecular weight polymer.Int J Biol Macromol. 2023 Dec 1;252:126382. doi: 10.1016/j.ijbiomac.2023.126382. Epub 2023 Aug 16. Int J Biol Macromol. 2023. PMID: 37595716
-
Generation and Use of Lignin-g-AMPS in Extended DLVO Theory for Evaluating the Flocculation of Colloidal Particles.ACS Omega. 2020 Aug 11;5(33):21032-21041. doi: 10.1021/acsomega.0c02598. eCollection 2020 Aug 25. ACS Omega. 2020. PMID: 32875240 Free PMC article.
-
Dual lignin-derived polymeric systems for hazardous ion removals.J Hazard Mater. 2021 Sep 5;417:125970. doi: 10.1016/j.jhazmat.2021.125970. Epub 2021 Apr 29. J Hazard Mater. 2021. PMID: 33975163
Cited by
-
Production and Application of Triblock Hydrolysis Lignin-Based Anionic Copolymers in Aqueous Systems.ACS Omega. 2021 Feb 23;6(9):6393-6403. doi: 10.1021/acsomega.0c06344. eCollection 2021 Mar 9. ACS Omega. 2021. PMID: 33718730 Free PMC article.
-
Lignin-g-poly(acrylamide)-g-poly(diallyldimethyl- ammonium chloride): Synthesis, Characterization and Applications.ChemistryOpen. 2018 Aug 27;7(8):645-658. doi: 10.1002/open.201800105. eCollection 2018 Aug. ChemistryOpen. 2018. PMID: 30155399 Free PMC article.
-
Sulfonated Lignin-g-Styrene Polymer: Production and Characterization.Polymers (Basel). 2018 Aug 19;10(8):928. doi: 10.3390/polym10080928. Polymers (Basel). 2018. PMID: 30960853 Free PMC article.
-
In Situ Copolymerization Studies of Lignin, Acrylamide, and Diallyldimethylammonium Chloride: Mechanism, Kinetics, and Rheology.ACS Omega. 2023 Jul 21;8(30):27156-27169. doi: 10.1021/acsomega.3c02296. eCollection 2023 Aug 1. ACS Omega. 2023. PMID: 37546615 Free PMC article.
-
Enhanced Performance of Lignin Recovery with a Carbon Dioxide Acidification Method.ACS Omega. 2023 Feb 15;8(8):7438-7447. doi: 10.1021/acsomega.2c06153. eCollection 2023 Feb 28. ACS Omega. 2023. PMID: 36872975 Free PMC article.
References
-
- Zhang X. Tu M. B. Paice M. G. BioEnergy Res. 2011;4:246–257. doi: 10.1007/s12155-011-9147-1. - DOI
-
- Stewart D. Ind. Crops Prod. 2008;27:202–207. doi: 10.1016/j.indcrop.2007.07.008. - DOI
-
- Isikgor F. H. Becer C. R. Polym. Chem. 2015;6:4497–4559. doi: 10.1039/C5PY00263J. - DOI
-
- Fatehi P. Curr. Org. Chem. 2013;17:1569. doi: 10.2174/1385272811317150002. - DOI
LinkOut - more resources
Full Text Sources

