Electroless plating of a Sn-Ni/graphite sheet composite with improved cyclability as an anode material for lithium ion batteries
- PMID: 35539458
- PMCID: PMC9079991
- DOI: 10.1039/c8ra01940a
Electroless plating of a Sn-Ni/graphite sheet composite with improved cyclability as an anode material for lithium ion batteries
Abstract
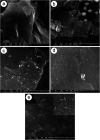
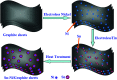
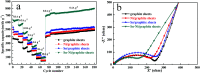
A Sn-Ni/graphite sheet composite is synthesized by a simple electroless plating method as an anode material for lithium ion batteries (LIBs). The microstructure and electrochemical properties of the composite are characterized by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), cyclic voltammetry (CV), and AC impedance spectroscopy. The results show that the as-prepared composite has Sn-Ni nanoparticles around 100 nm in size, where metallic Ni acts as an "anchor" to fix metallic Sn. The reunion phenomenon of Sn is alleviated by adding metallic Ni between the metallic Sn and graphite sheets. The Sn-Ni/graphite sheet electrode exhibits a good rate performance with a capability of 637.4, 586.3, 466.7, 371.5, 273.6, 165.3 and 97.3 mA h g-1 at a current density of 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10 A g-1, respectively. The good electrical conductivity of Ni, high specific capacity of Sn and excellent cycling capability of the graphite sheets have a synergistic effect and are the main reasons behind the superior electrochemical performance. Furthermore, the as-prepared composite exhibits excellent lithium storage capacity and the reversible capacity increased as the cycle number increased.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Cao K. Li P. Zhang Y. Chen T. Wang X. Zhang S. Liu J. Wang H. Nano Energy. 2017;40:187–194. doi: 10.1016/j.nanoen.2017.07.042. - DOI
-
- Chen J. Yano K. ACS Appl. Mater. Interfaces. 2013;5:7682–7687. - PubMed
-
- Cheng Y. Yi Z. Wang C. Wu Y. Wang L. Chem. Eng. J. 2017;330:1035–1043. doi: 10.1016/j.cej.2017.08.066. - DOI
-
- Leng J. Wang Z. Li X. Guo H. Li H. Shih K. Yan G. Wang J. J. Mater. Chem. A. 2017;5:14996–15001.
-
- Li T. Li X. Wang Z. Guo H. Li Y. Wang J. J. Mater. Chem. A. 2017;5:13469–13474.
LinkOut - more resources
Full Text Sources

