Diastereoselective construction of 4-indole substituted chromans bearing a ketal motif through a three-component Friedel-Crafts alkylation/ketalization sequence
- PMID: 35539466
- PMCID: PMC9080074
- DOI: 10.1039/c8ra02481b
Diastereoselective construction of 4-indole substituted chromans bearing a ketal motif through a three-component Friedel-Crafts alkylation/ketalization sequence
Abstract
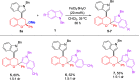
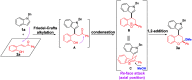
The first TfOH-catalyzed three-component Friedel-Crafts alkylation/ketalization sequence of indoles, alcohols and ortho-hydroxychalcones was developed to afford a wide range of 4-indole substituted chromans bearing a ketal motif in 77-99% yields. Notably, only a simple filtration was needed to purify them. By altering methanol to CHCl3, 2,4-bisindole substituted chroman with the same indole substituent at the C2 and C4 positions was afforded. Moreover, 2,4-bisindole substituted chromans with different indole substituents could be obtained by treatment of 4-indole monosubstituted chromans with another indole molecule.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
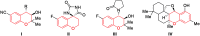

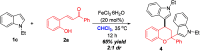
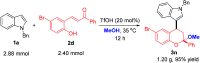

References
-
-
For selected reviews:
- Touré B. B. Hall D. G. Chem. Rev. 2009;109:4439. doi: 10.1021/cr800296p. - DOI - PubMed
- Sunderhaus J. D. Martin S. F. Chem.–Eur. J. 2009;15:1300. doi: 10.1002/chem.200802140. - DOI - PMC - PubMed
- Jiang B. Rajale T. Wever W. Tu S.-J. Li G.-G. Chem.–Asian J. 2010;5:2318. doi: 10.1002/asia.201000310. - DOI - PubMed
- Wu J. Shi F. Gong L.-Z. Acc. Chem. Res. 2011;44:1156. doi: 10.1021/ar2000343. - DOI - PubMed
- Cioc R. C. Ruijter E. Orru R. V. A. Green Chem. 2014;16:1958. doi: 10.1039/C4GC00013G. - DOI
- Hassan S. Müller T. J. J. Adv. Synth. Catal. 2015;357:617. doi: 10.1002/adsc.201400904. - DOI
-
-
-
For selected reviews:
- Pratap R. Ram V. J. Chem. Rev. 2014;114:10476. doi: 10.1021/cr500075s. - DOI - PubMed
-
For selected examples:
- Hiessböck R. Wolf C. Richter E. Hitzler M. Chiba P. Kratzel M. Ecker G. J. Med. Chem. 1999;42:1921. doi: 10.1021/jm980517+. - DOI - PubMed
- Nicolaou K. C. Pfefferkorn J. A. Roecker A. J. Cao C.-Q. Barluenga S. Mitchell H. J. J. Am. Chem. Soc. 2000;122:9939. doi: 10.1021/ja002033k. - DOI
- Trost B. M. Shen H.-C. Surivet J.-P. J. Am. Chem. Soc. 2004;126:12565. doi: 10.1021/ja048084p. - DOI - PubMed
- Kumar S. Deshpande S. Chandra V. Kitchlu S. Dwivedi A. Nayak V. L. Konwar R. Prabhakar Y. S. Sahu D. P. Bioorg. Med. Chem. 2009;17:6832. doi: 10.1016/j.bmc.2009.08.034. - DOI - PubMed
- Ketcham J. M. Volchkov I. Chen T.-Y. Blumberg P. M. Kedei N. Lewin N. E. Krische M. J. J. Am. Chem. Soc. 2016;138:13415. doi: 10.1021/jacs.6b08695. - DOI - PMC - PubMed
- Kumar M. Chauhan P. Valkonen A. Rissanen K. Enders D. Org. Lett. 2017;19:3025. doi: 10.1021/acs.orglett.7b01322. - DOI - PubMed
-
-
-
For selected reviews:
- Saxton J. E. Nat. Prod. Rep. 1997;14:559. doi: 10.1039/NP9971400559. - DOI
- Somei M. Yamada F. Nat. Prod. Rep. 2004;21:278. doi: 10.1039/B212257J. - DOI - PubMed
- Kawasaki T. Higuchi K. Nat. Prod. Rep. 2005;22:761. doi: 10.1039/B502162F. - DOI - PubMed
- O'Connor S. E. Maresh J. J. Nat. Prod. Rep. 2006;23:532. doi: 10.1039/B512615K. - DOI - PubMed
- Humphrey G. R. Kuethe J. T. Chem. Rev. 2006;106:2875. doi: 10.1021/cr0505270. - DOI - PubMed
- Higuchi K. Kawasaki T. Nat. Prod. Rep. 2007;24:843. doi: 10.1039/B516351J. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Miscellaneous

