Silver ions blocking crystallization of guanosine-based hydrogel for potential antimicrobial applications
- PMID: 35539473
- PMCID: PMC9080096
- DOI: 10.1039/c8ra02500b
Silver ions blocking crystallization of guanosine-based hydrogel for potential antimicrobial applications
Abstract
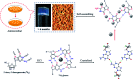
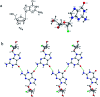
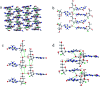
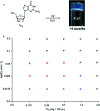
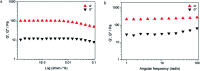
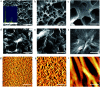
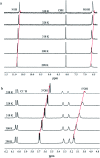
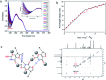
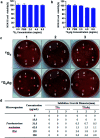
In this work, the detailed crystallization process of 2'-deoxy-2'-fluoroguanosine (FGd) hydrogel has been studied using single crystal X-ray diffraction, variable-temperature nuclear magnetic resonance (VT-NMR), and scanning electron microscopy (SEM). Both solid and solution results indicated that the K+-mediated G-quartet structures were unstable and easily resulted in the breakdown of the hydrogel to form linear ribbon structures by forming mimic reverse Watson-Crick base pairs between the two faces with an intermolecular hydrogen-bond (N10H-O11). Accordingly, Ag+ was introduced to block the crystallization of FGd to form long lifetime stable supramolecular hydrogel (>6 months) and possible silver-ions-mediated base pair motifs were suggested via NMR, UV, and mass spectroscopy (MS) in combination with powder X-ray diffraction (PXRD) and circular dichroism spectroscopy (CD). Furthermore, FGdAg hydrogel exhibited low toxicity for normal oral keratinocyte cells (NOK-SI) and good antibacterial activities for Fusobacterium nucleatum in vitro.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Developing a Self-Healing Supramolecular Nucleoside Hydrogel Based on Guanosine and Isoguanosine.Chem Asian J. 2018 Jun 19. doi: 10.1002/asia.201800788. Online ahead of print. Chem Asian J. 2018. PMID: 29920951
-
Silver(I)-Mediated Base Pairs in DNA Sequences Containing 7-Deazaguanine/Cytosine: towards DNA with Entirely Metallated Watson-Crick Base Pairs.Chemistry. 2018 Mar 26;24(18):4583-4589. doi: 10.1002/chem.201705131. Epub 2018 Jan 25. Chemistry. 2018. PMID: 29226453
-
Highly Stable Double-Stranded DNA Containing Sequential Silver(I)-Mediated 7-Deazaadenine/Thymine Watson-Crick Base Pairs.Angew Chem Int Ed Engl. 2016 May 17;55(21):6170-4. doi: 10.1002/anie.201600924. Epub 2016 Mar 23. Angew Chem Int Ed Engl. 2016. PMID: 27005864
-
Ag(I)-mediated homo and hetero pairs of guanosine and cytidine: monitoring by circular dichroism spectroscopy.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Jan 24;118:221-7. doi: 10.1016/j.saa.2013.08.101. Epub 2013 Aug 31. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24051294
-
Probing hydrogen bonding and ion-carbonyl interactions by solid-state 17O NMR spectroscopy: G-ribbon and G-quartet.J Am Chem Soc. 2007 Feb 28;129(8):2398-407. doi: 10.1021/ja067991m. Epub 2007 Feb 2. J Am Chem Soc. 2007. PMID: 17269776
Cited by
-
Sulfonamide as amide isostere for fine-tuning the gelation properties of physical gels.RSC Adv. 2020 Mar 20;10(19):11481-11492. doi: 10.1039/d0ra00943a. eCollection 2020 Mar 16. RSC Adv. 2020. PMID: 35495355 Free PMC article.
-
Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges.Exploration (Beijing). 2023 Dec 22;4(1):20230092. doi: 10.1002/EXP.20230092. eCollection 2024 Feb. Exploration (Beijing). 2023. PMID: 38854496 Free PMC article. Review.
-
Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering.Biomater Res. 2018 Sep 26;22:27. doi: 10.1186/s40824-018-0138-6. eCollection 2018. Biomater Res. 2018. PMID: 30275970 Free PMC article. Review.
-
The Development and Lifetime Stability Improvement of Guanosine-Based Supramolecular Hydrogels through Optimized Structure.Biomed Res Int. 2019 Jun 13;2019:6258248. doi: 10.1155/2019/6258248. eCollection 2019. Biomed Res Int. 2019. PMID: 31312660 Free PMC article. Review.
References
-
- Das D. Pal S. RSC Adv. 2015;5:25014–25050. doi: 10.1039/C4RA16103C. - DOI
LinkOut - more resources
Full Text Sources

