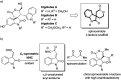
A C1-symmetric N-heterocyclic carbene catalysed oxidative spiroannulation of isatin-derived enals: highly enantioselective synthesis of spirooxindole δ-lactones
- PMID: 35539478
- PMCID: PMC9080099
- DOI: 10.1039/c8ra02009d
A C1-symmetric N-heterocyclic carbene catalysed oxidative spiroannulation of isatin-derived enals: highly enantioselective synthesis of spirooxindole δ-lactones
Abstract
A C1-symmetric N-heterocyclic carbene (NHC)-catalysed activation of isatin-derived enals under oxidative conditions was achieved. The in situ generated α,β-unsaturated acyl azolium species was efficiently trapped by 1,3-dicarbonyl compounds via a Michael addition/spiroannualtion cascade, delivering a series of synthetically important spirooxindole δ-lactones with up to 96% enantioselectivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles.Acc Chem Res. 2019 Feb 19;52(2):425-436. doi: 10.1021/acs.accounts.8b00550. Epub 2019 Jan 17. Acc Chem Res. 2019. PMID: 30653296
-
Enantioselective access to 3,3'-disubstituted oxindole derivatives by N-heterocyclic carbene catalysis.Org Biomol Chem. 2025 Jun 11;23(23):5527-5532. doi: 10.1039/d5ob00374a. Org Biomol Chem. 2025. PMID: 40391563
-
N-Heterocyclic Carbene-Catalyzed Michael-Michael-Lactonization Cascade for the Enantioselective Synthesis of Tricyclic δ-Lactones.Org Lett. 2018 May 18;20(10):2952-2955. doi: 10.1021/acs.orglett.8b00998. Epub 2018 May 3. Org Lett. 2018. PMID: 29722983
-
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations.Angew Chem Int Ed Engl. 2018 Apr 3;57(15):3862-3873. doi: 10.1002/anie.201709684. Epub 2018 Mar 9. Angew Chem Int Ed Engl. 2018. PMID: 29136320 Review.
-
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.Acc Chem Res. 2020 Mar 17;53(3):690-702. doi: 10.1021/acs.accounts.9b00635. Epub 2020 Mar 6. Acc Chem Res. 2020. PMID: 32142245 Review.
Cited by
-
Recent Advances in the Synthesis of 3,4-Dihydropyran-2-Ones Organocatalyzed by N-Heterocyclic Carbenes.Molecules. 2023 Apr 26;28(9):3743. doi: 10.3390/molecules28093743. Molecules. 2023. PMID: 37175154 Free PMC article. Review.
References
-
-
For selected reviews, see:
- Marti C. Carreira E. M. Eur. J. Org. Chem. 2003:2209. doi: 10.1002/ejoc.200300050. - DOI
- Galliford C. V. Scheidt K. A. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. - DOI - PubMed
- Dalpozzo R. Bartoli G. Bencivenni G. Chem. Soc. Rev. 2012;41:7247. doi: 10.1039/C2CS35100E. - DOI - PubMed
- Ball-Jones N. R. Badillo J. J. Franz A. K. Org. Biomol. Chem. 2012;10:5165. doi: 10.1039/C2OB25184A. - DOI - PubMed
-
-
-
For selected examples, see:
- Chen X. H. Wei Q. Luo S. W. Xiao H. Gong L. Z. J. Am. Chem. Soc. 2009;131:13819. doi: 10.1021/ja905302f. - DOI - PubMed
- Antonchick A. P. Gerding-Reimers C. Catarinella M. Schürmann M. Preut H. Ziegler S. Rauh D. Waldmann H. Nat. Chem. 2010;2:735. doi: 10.1038/nchem.730. - DOI - PubMed
- Tan B. Candeias N. R. Barbas III C. F. Nat. Chem. 2011;3:473. doi: 10.1038/nchem.1039. - DOI - PubMed
- Liu Y. Nappi M. Arceo E. Vera S. Melchiorre P. J. Am. Chem. Soc. 2011;133:15212. doi: 10.1021/ja206517s. - DOI - PubMed
- Zhan G. Shi M. L. He Q. Lin W. J. Ouyang Q. Du W. Chen Y. C. Angew. Chem., Int. Ed. 2016;55:2147. doi: 10.1002/anie.201510825. - DOI - PubMed
- Han X. Chan W. L. Yao W. Wang Y. Lu Y. Angew. Chem., Int. Ed. 2016;55:6492. doi: 10.1002/anie.201600453. - DOI - PubMed
-
-
- Chen Y. G. Wu J. C. Chen G. Y. Han C. R. Song X. P. Chem. Biodiversity. 2011;8:1958. doi: 10.1002/cbdv.201000325. - DOI - PubMed
- Ma S. S. Mei W. L. Guo Z. K. Liu S. B. Zhao Y. X. Yang D. L. Zeng Y. B. Jiang B. Dai H. F. Org. Lett. 2013;15:1492. doi: 10.1021/ol4002619. - DOI - PubMed
- Huang J. Z. Zhang C. L. Zhu Y. F. Li L. L. Chen D. F. Han Z. Y. Gong L. Z. Chem.–Eur. J. 2015;21:8389. doi: 10.1002/chem.201500349. - DOI - PubMed
-
-
For selected reviews, see:
- Enders D. Niemeier O. Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z. - DOI - PubMed
- Nair V. Vellalath S. Babu B. P. Chem. Soc. Rev. 2008;37:2691. doi: 10.1039/B719083M. - DOI - PubMed
- Grossmann A. Enders D. Angew. Chem., Int. Ed. 2012;51:314. doi: 10.1002/anie.201105415. - DOI - PubMed
- Izquierdo J. Hutson G. E. Cohen D. T. Scheidt K. A. Angew. Chem., Int. Ed. 2012;51:11686. doi: 10.1002/anie.201203704. - DOI - PMC - PubMed
- Bugaut X. Glorius F. Chem. Soc. Rev. 2012;41:3511. doi: 10.1039/C2CS15333E. - DOI - PubMed
- Ryan S. J. Candish L. Lupton D. W. Chem. Soc. Rev. 2013;42:4906. doi: 10.1039/C3CS35522E. - DOI - PubMed
- Hopkinson M. N. Richter C. Schedler M. Glorius F. Nature. 2014;510:485. doi: 10.1038/nature13384. - DOI - PubMed
- Mahatthananchai J. Bode J. W. Acc. Chem. Res. 2014;47:696. doi: 10.1021/ar400239v. - DOI - PubMed
- Flanigan D. M. Romanov-Michailidis F. White N. A. Rovis T. Chem. Rev. 2015;115:9307. doi: 10.1021/acs.chemrev.5b00060. - DOI - PMC - PubMed
- Wang Y. Wei D. Zhang W. ChemCatChem. 2018;10:338. doi: 10.1002/cctc.201701119. - DOI
- Chen X. Y. Liu Q. Chauhan P. Enders D. Angew. Chem., Int. Ed. 2018;57:3862. doi: 10.1002/anie.201709684. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources