Catalyst- and solvent-free approach to 2-arylated quinolines via [5 + 1] annulation of 2-methylquinolines with diynones
- PMID: 35539512
- PMCID: PMC9077778
- DOI: 10.1039/c7ra12716b
Catalyst- and solvent-free approach to 2-arylated quinolines via [5 + 1] annulation of 2-methylquinolines with diynones
Abstract
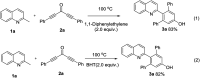
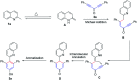
A novel route for the synthesis of 2-arylated quinolines through a [5 + 1] annulation directly from 2-methylquinolines and diynones under catalyst-free and solvent-free conditions was disclosed. This synthetic process was atom-economic, with good tolerance of a broad range of functional groups, and with great practical worth.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Catalyst-Free Synthesis of Pyrrolo[1,2-a]quinolines via Dehydration/[3 + 2] Cycloaddition Directly from 2-Methylquinolines, Aldehydes, and Alkynoates.J Org Chem. 2017 Apr 21;82(8):4289-4296. doi: 10.1021/acs.joc.7b00280. Epub 2017 Apr 3. J Org Chem. 2017. PMID: 28349695
-
An efficient approach to quinolines via Friedländer synthesis catalyzed by cuprous triflate.Chem Pharm Bull (Tokyo). 2010 Feb;58(2):212-3. doi: 10.1248/cpb.58.212. Chem Pharm Bull (Tokyo). 2010. PMID: 20118581
-
Catalyst- and Additive-Free Annulation of Ynediones and (Iso)Quinoline N-Oxides: An Approach to Synthesis of Pyrrolo[2,1-a]Isoquinolines and Pyrrolo[1,2-a]Quinolines.J Org Chem. 2021 Jan 1;86(1):169-177. doi: 10.1021/acs.joc.0c01932. Epub 2020 Nov 30. J Org Chem. 2021. PMID: 33252226
-
Zinc triflate: a highly efficient reusable catalyst in the synthesis of functionalized quinolines via Friedlander annulation.Mol Divers. 2010 Nov;14(4):841-6. doi: 10.1007/s11030-009-9214-0. Epub 2009 Dec 5. Mol Divers. 2010. PMID: 19960367
-
Unexpected Annulation between 2-Aminobenzyl Alcohols and Benzaldehydes in the Presence of DMSO: Regioselective Synthesis of Substituted Quinolines.J Org Chem. 2021 Nov 5;86(21):15228-15241. doi: 10.1021/acs.joc.1c01850. Epub 2021 Oct 11. J Org Chem. 2021. PMID: 34632772
Cited by
-
New Trends in C-C Cross-Coupling Reactions: The Use of Unconventional Conditions.Molecules. 2020 Nov 24;25(23):5506. doi: 10.3390/molecules25235506. Molecules. 2020. PMID: 33255429 Free PMC article. Review.
References
-
- Hitora Y. Takada K. Ise Y. Okada S. Matsunaga S. J. Nat. Prod. 2016;79:2973. doi: 10.1021/acs.jnatprod.6b00710. - DOI - PubMed
- Fan H. Peng J.-G. Hamann M. T. Hu J.-F. Chem. Rev. 2008;108:264. doi: 10.1021/cr078199m. - DOI - PMC - PubMed
- Boucherle B. Haudecoeur R. Queiroz E. F. de Waard M. Wolfender J.-L. Robins R. J. Boumendjel A. Nat. Prod. Rep. 2016;33:1034. doi: 10.1039/C6NP00039H. - DOI - PubMed
- Tang H.-T. Xiong K. Li R.-H. Ding Z.-C. Zhan Z.-P. Org. Lett. 2015;17:326. doi: 10.1021/ol503437n. - DOI - PubMed
- Wen J.-J. Tang H.-T. Xiong K. Ding Z.-C. Zhan Z.-P. Org. Lett. 2014;16:5940. doi: 10.1021/ol502968c. - DOI - PubMed
- Tang X. Zhu Z. Qi C. Wu W. Jiang H. Org. Lett. 2016;18:180. doi: 10.1021/acs.orglett.5b03188. - DOI - PubMed
- Li P. Zhang X. Fan X. J. Org. Chem. 2015;80:7508. doi: 10.1021/acs.joc.5b01092. - DOI - PubMed
- Zhang S.-S. Wu J.-Q. Liu X. Wang H. ACS Catal. 2015;5:210. doi: 10.1021/cs501601c. - DOI
-
- Gutekunst W. R. Gianatassio R. Baran P. S. Angew. Chem., Int. Ed. 2012;124:7625. doi: 10.1002/ange.201203897. - DOI - PMC - PubMed
- Arockiam P. B. Bruneau C. Dixneuf P. H. Chem. Rev. 2012;112:5879. doi: 10.1021/cr300153j. - DOI - PubMed
- Cho S. H. Kim J. Y. Kwak J. Chang S. Chem. Soc. Rev. 2011;40:5068. doi: 10.1039/C1CS15082K. - DOI - PubMed
- Chen X. Engle K. M. Wang D.-H. Yu J.-Q. Angew. Chem., Int. Ed. 2009;121:5196. doi: 10.1002/ange.200806273. - DOI - PubMed
-
- Quirk J. Thornton M. Kirkpatrick P. Nature. 2003;2:769. - PubMed
-
- Capdeville R. Buchdunger E. Zimmermann J. Matter A. Nature. 2002;1:493. - PubMed
LinkOut - more resources
Full Text Sources