Green synthesis of new pyrrolidine-fused spirooxindoles via three-component domino reaction in EtOH/H2O
- PMID: 35539589
- PMCID: PMC9078157
- DOI: 10.1039/c7ra13207g
Green synthesis of new pyrrolidine-fused spirooxindoles via three-component domino reaction in EtOH/H2O
Abstract
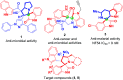
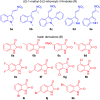
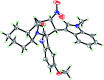
An efficient, green and sustainable approach for the synthesis of novel polycyclic pyrrolidine-fused spirooxindole compounds was developed. The synthesis included a one-pot, three-component, domino reaction of (E)-3-(2-nitrovinyl)-indoles, isatins and chiral polycyclic α-amino acids under catalyst-free conditions at room temperature in EtOH-H2O. The salient features of this methodology are eco-friendliness, high yields and the ease of obtaining target compounds without the involvement of toxic solvents and column chromatography. These novel polycyclic pyrrolidine-fused spirooxindoles provide a collection of structurally diverse compounds that show promise for future bioassays and medical treatments.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles.Int J Mol Sci. 2023 Feb 13;24(4):3771. doi: 10.3390/ijms24043771. Int J Mol Sci. 2023. PMID: 36835183 Free PMC article.
-
A green synthetic approach toward the synthesis of structurally diverse spirooxindole derivative libraries under catalyst-free conditions.Mol Divers. 2017 May;21(2):325-337. doi: 10.1007/s11030-017-9728-9. Epub 2017 Feb 11. Mol Divers. 2017. PMID: 28190223
-
Magnetic, acidic, ionic liquid-catalyzed one-pot synthesis of spirooxindoles.ACS Comb Sci. 2013 Sep 9;15(9):512-8. doi: 10.1021/co400080z. Epub 2013 Aug 23. ACS Comb Sci. 2013. PMID: 23971413
-
Copper ferrite nanoparticles: an efficient and reusable nanocatalyst for a green one-pot, three-component synthesis of spirooxindoles in water.ACS Comb Sci. 2013 Oct 14;15(10):530-4. doi: 10.1021/co400057h. Epub 2013 Sep 19. ACS Comb Sci. 2013. PMID: 24050156
-
One-pot, three-component synthesis of spirooxindoles catalyzed by ZnO nano-rods in solvent-free conditions.Comb Chem High Throughput Screen. 2012 Dec;15(10):826-34. doi: 10.2174/138620712803901144. Comb Chem High Throughput Screen. 2012. PMID: 23140190
Cited by
-
Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles.Int J Mol Sci. 2023 Feb 13;24(4):3771. doi: 10.3390/ijms24043771. Int J Mol Sci. 2023. PMID: 36835183 Free PMC article.
-
Activator free diastereoselective 1,3-dipolar cycloaddition: a quick access to coumarin based spiro multi heterocyclic adducts.RSC Adv. 2021 Sep 8;11(48):29934-29938. doi: 10.1039/d1ra05070b. eCollection 2021 Sep 6. RSC Adv. 2021. PMID: 35480285 Free PMC article.
-
Total synthesis of isatindigotindoline C.Org Biomol Chem. 2020 Mar 18;18(11):2051-2053. doi: 10.1039/d0ob00270d. Org Biomol Chem. 2020. PMID: 32141462 Free PMC article.
-
Synthesis of new functionalized thiazolo pyridine-fused and thiazolo pyridopyrimidine-fused spirooxindoles via one-pot reactions.Heliyon. 2020 Mar 31;6(3):e03687. doi: 10.1016/j.heliyon.2020.e03687. eCollection 2020 Mar. Heliyon. 2020. PMID: 32258502 Free PMC article.
-
Recent developments in green approaches for sustainable synthesis of indole-derived scaffolds.Mol Divers. 2022 Dec;26(6):3411-3445. doi: 10.1007/s11030-021-10376-3. Epub 2022 Jan 15. Mol Divers. 2022. PMID: 35031935 Review.
References
-
- Khoobi M. Delshad T. M. Vosooghi M. Alipour M. Hamadi H. Alipour E. Hamedani M. P. Safaei Z. Foroumadi A. Shafiee A. J. Magn. Magn. Mater. 2015;375:217–226. doi: 10.1016/j.jmmm.2014.09.044. - DOI
- Khazaei A. Zolfigol M. A. Karimitabar F. Nikokar I. Moosavi-Zare A. R. RSC Adv. 2015;5:71402–71412. doi: 10.1039/C5RA10730J. - DOI
- Ma Y.-L. Wang K.-M. Huang R. Lin J. Yan S.-J. Green Chem. 2017;19:3574–3584. doi: 10.1039/C7GC01435J. - DOI
- Wu H. Chen X.-M. Wan Y. Ye L. Xin H.-Q. Xu H.-H. Yue C.-H. Pang L.-L. Ma R. Shi D.-Q. Tetrahedron Lett. 2009;50:1062–1065. doi: 10.1016/j.tetlet.2008.12.067. - DOI
- Yan L.-J. Wang J.-L. Xu D. Burgess K. S. Zhu A.-F. Rao Y.-Y. Chen X.-B. Wang Y.-C. ChemistrySelect. 2018;3:662–665. doi: 10.1002/slct.201702188. - DOI
-
- Volla C. M. R. Atodiresei I. Rueping M. Chem. Rev. 2014;114:2390–2431. doi: 10.1021/cr400215u. - DOI - PubMed
- Dömling A. Wang W. Wang K. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. - DOI - PMC - PubMed
- Trost B. M. Frontier A. J. J. Am. Chem. Soc. 2000;122:11727–11728. doi: 10.1021/ja0022268. - DOI
- Trost B. M. Gutierrez A. C. Livingston R. C. Org. Lett. 2009;11:2539–2542. doi: 10.1021/ol9007876. - DOI - PMC - PubMed
- Jiang B. Tu S.-J. Kaur P. Wever W. Li G.-G. J. Am. Chem. Soc. 2009;131:11660–11661. doi: 10.1021/ja904011s. - DOI - PubMed
- Jiang B. Yi M.-S. Shi F. Tu S.-J. Pindi S. McDowell P. Li G. Chem. Commun. 2012;48:808–810. doi: 10.1039/C1CC15913E. - DOI - PMC - PubMed
- Sun J. Sun Y. Gong H. Xie Y.-J. Yan C.-G. Org. Lett. 2012;14:5172–5175. doi: 10.1021/ol302530m. - DOI - PubMed
- Feng X. Wang Q. Lin W. Dou G.-L. Huang Z.-B. Shi D.-Q. Org. Lett. 2013;15:2542–2545. doi: 10.1021/ol4010382. - DOI - PubMed
LinkOut - more resources
Full Text Sources

