One-pot synthesis of amine-functionalized graphene oxide by microwave-assisted reactions: an outstanding alternative for supporting materials in supercapacitors
- PMID: 35539592
- PMCID: PMC9078217
- DOI: 10.1039/c7ra13514a
One-pot synthesis of amine-functionalized graphene oxide by microwave-assisted reactions: an outstanding alternative for supporting materials in supercapacitors
Abstract
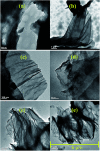
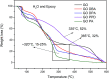
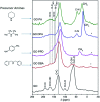
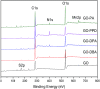
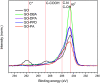
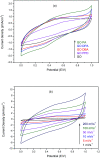
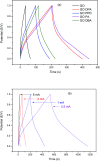
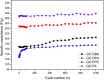
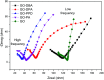
A simple and straightforward method using microwave-assisted reactions is presented for the functionalization of graphene oxide with aromatic and non-aromatic amines, notedly dibenzylamine (DBA), p-phenylenediamine (PPD), diisopropylamine (DPA) and piperidine (PA). The as-synthesized amine-functionalized graphene oxide materials (amine-GO) were characterized using spectroscopic techniques including XRD, FTIR, 13C NMR, XPS, TEM for imaging and thermogravimetric analysis (TGA). The characterization confirmed the functionalization for all amines, reaching relatively high surface nitrogen atomic concentrations of up to 8.8%. The investigations of electrochemical behavior for the amine-GOs show the significant improvement in GO's electrochemical properties through amine functionalization, exhibiting long life cycle stability and reaching specific capacitance values of up to 290 F g-1 and 260 F g-1 for GO-PA and GO-DPA samples, respectively, confirming their potential application as alternative supporting materials in supercapacitors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- He G. Li J. Chen H. Shi J. Sun X. Chen S. Wang X. Mater. Lett. 2012;82:61–63. doi: 10.1016/j.matlet.2012.05.048. - DOI
-
- Liu T. Chai H. Jia D. Su Y. Wang T. Zhou W. Electrochim. Acta. 2015;180:998–1006. doi: 10.1016/j.electacta.2015.07.175. - DOI
-
- Hussain S. Liu T. Javed M. S. Aslam N. Shaheen N. Zhao S. Zeng W. Wang J. Ceram. Int. 2016;42:11851–11857. doi: 10.1016/j.ceramint.2016.04.107. - DOI
-
- Guan C. Liu X. Ren W. Li X. Cheng C. Wang J. Adv. Energy Mater. 2017;7(12):1602391. doi: 10.1002/aenm.201602391. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

