Cyclization of secondarily structured oligonucleotides to single-stranded rings by using Taq DNA ligase at high temperatures
- PMID: 35539641
- PMCID: PMC9080623
- DOI: 10.1039/c8ra02804d
Cyclization of secondarily structured oligonucleotides to single-stranded rings by using Taq DNA ligase at high temperatures
Abstract
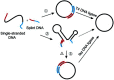
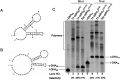
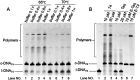
Single-stranded DNA rings play important roles in nanoarchitectures, molecular machines, DNA detection, etc. Although T4 DNA ligase has been widely employed to cyclize single-stranded oligonucleotides into rings, the cyclization efficiency is very low when the oligonucleotides (l-DNAs) take complicated secondary structures at ambient temperatures. In the present study, this problem has been solved by using Thermus aquaticus DNA ligase (Taq DNA ligase) at higher temperatures (65 and 70 °C) where the secondary structures are less stable or completely destroyed. This method is based on our new finding that this ligase successfully functions even when the splint strand is short and forms no stable duplex with l-DNA (at least in the absence of the enzyme). In order to increase the efficiency of cyclization, various operation factors (lengths and sequences of splint, as well as the size of the DNA ring) have been investigated. Based on these results, DNA rings have been successfully synthesized from secondarily structured oligonucleotides in high yields and high selectivity. The present methodology is applicable to the preparation of versatile DNA rings involving complicated secondary structures, which should show novel properties and greatly widen the scope of DNA-based nanotechnology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
None declared.
Figures




References
-
- Komiyama M. Yoshimoto K. Sisido M. Ariga K. Bull. Chem. Soc. Jpn. 2017;90:967–1004. doi: 10.1246/bcsj.20170156. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

