Decontamination of radioactive cesium ions using ordered mesoporous monetite
- PMID: 35539644
- PMCID: PMC9080637
- DOI: 10.1039/c8ra02707b
Decontamination of radioactive cesium ions using ordered mesoporous monetite
Abstract
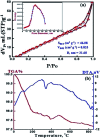
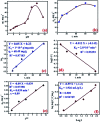
We report herein the fabrication of an environmentally friendly, low-cost and efficient nanostructured mesoporous monetite plate-like mineral (CaHPO4) as an adsorbent for removal of radioactive cesium ions from aqueous solutions. The phase and textural features of the synthesized mesoporous monetite were well characterized by XRD, FTIR, SEM, HRTEM, DLS, TGA/TDA, and N2 adsorption/desorption techniques. The results indicate that the cesium ions were effectively adsorbed by the mesoporous monetite ion-exchanger (MMT-IEX) above pH 9.0. Different kinetic and isotherm models were applied to characterize the cesium adsorption process. The fabricated monetite exhibited a monolayer adsorption capacity up to 60.33 mg g-1 at pH of 9.5. The collected data revealed the higher ability of CaHPO4 for the removal of Cs(i) from aqueous media in an efficient way.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Alby D. Charnay C. Heran M. Prelot B. Zajac J. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity-A review. J. Hazard. Mater. 2018;344:511–530. doi: 10.1016/j.jhazmat.2017.10.047. - DOI - PubMed
-
- Vincent C. Hertz A. Vincent T. Barré Y. Guibal E. Immobilization of inorganic ion-exchanger into biopolymer foams – Application to cesium sorption. Chem. Eng. J. 2014;236:202–211. doi: 10.1016/j.cej.2013.09.087. - DOI
-
- Lee D. A. Solvent extraction of cesium and rubidium triphenylcyanoboron. J. Inorg. Nucl. Chem. 1972;34:2895–2901. doi: 10.1016/0022-1902(72)80597-9. - DOI
LinkOut - more resources
Full Text Sources

