Supramolecular hydrogels encapsulating bioengineered mesenchymal stem cells for ischemic therapy
- PMID: 35539688
- PMCID: PMC9080606
- DOI: 10.1039/c8ra00464a
Supramolecular hydrogels encapsulating bioengineered mesenchymal stem cells for ischemic therapy
Abstract
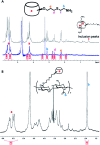
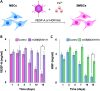
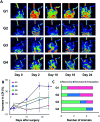
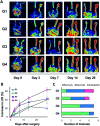
We developed supramolecular hyaluronate (HA) hydrogels to encapsulate genetically engineered mesenchymal stem cells (MSCs) for the treatment of limb ischemia. In vivo angiogenic factors could be produced stably by the bioengineered MSCs (BMSCs) within the supramolecular hydrogels showing effective vascular repair and enhanced blood perfusion.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict to declare.
Figures

Similar articles
-
Supramolecular Injectable Hyaluronate Hydrogels for Cartilage Tissue Regeneration.ACS Appl Bio Mater. 2020 Aug 17;3(8):5040-5047. doi: 10.1021/acsabm.0c00537. Epub 2020 Aug 6. ACS Appl Bio Mater. 2020. PMID: 35021681
-
Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model.Acta Biomater. 2019 Feb;85:94-105. doi: 10.1016/j.actbio.2018.12.015. Epub 2018 Dec 11. Acta Biomater. 2019. PMID: 30550934
-
A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization.Acta Biomater. 2016 May;36:86-98. doi: 10.1016/j.actbio.2016.03.002. Epub 2016 Mar 4. Acta Biomater. 2016. PMID: 26961805 Free PMC article.
-
Genetically engineered mesenchymal stem cell therapy using self-assembling supramolecular hydrogels.J Control Release. 2015 Dec 28;220(Pt A):119-129. doi: 10.1016/j.jconrel.2015.10.034. Epub 2015 Oct 17. J Control Release. 2015. PMID: 26485045 Review.
-
Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases.Int J Mol Sci. 2021 Dec 27;23(1):249. doi: 10.3390/ijms23010249. Int J Mol Sci. 2021. PMID: 35008675 Free PMC article. Review.
Cited by
-
Bioinspired Self-assembling Peptide Hydrogel with Proteoglycan-assisted Growth Factor Delivery for Therapeutic Angiogenesis.Theranostics. 2019 Sep 21;9(23):7072-7087. doi: 10.7150/thno.35803. eCollection 2019. Theranostics. 2019. PMID: 31660087 Free PMC article.
References
-
- Bussolino F. Di Renzo M. F. Ziche M. Bocchietto E. Olivero M. Naldini L. Gaudino G. Tamagnone L. Coffer A. Comoglio P. M. Di Renzo M. F. Ziche M. Bocchietto E. Olivero M. Naldini L. Gaudino G. Tamagnone L. Coffer A. Comoglio P. M. Di Renzo M. F. Ziche M. Bocchietto E. Olivero M. Naldini L. Gaudino G. Tamagnone L. Coffer A. Comoglio P. M. J. Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

