Synthesis of TiO2-ZnS nanocomposites via sacrificial template sulfidation and their ethanol gas-sensing performance
- PMID: 35539706
- PMCID: PMC9081378
- DOI: 10.1039/c8ra04157a
Synthesis of TiO2-ZnS nanocomposites via sacrificial template sulfidation and their ethanol gas-sensing performance
Abstract
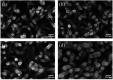
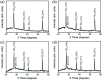
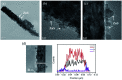
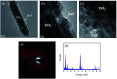
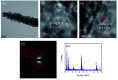
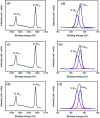
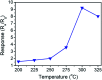
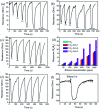
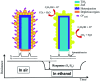
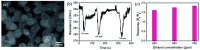
TiO2-ZnS core-shell composite nanorods were synthesized by using ZnO as a sacrificial shell layer in a hydrothermal reaction. ZnO thin films of different thicknesses were sputter-deposited onto the surfaces of TiO2 nanorods as templates for hydrothermally synthesizing TiO2-ZnS core-shell nanorods. Structural analysis revealed that crystalline TiO2-ZnS composite nanorods were formed without any residual ZnO phase after hydrothermal sulfidation in the composite nanorods. The thickness of the ZnO sacrificial shell layer affected the surface morphology and sulfur-related surface defect density in hydrothermally grown ZnS crystallites of TiO2-ZnS composite nanorods. Due to the distinctive core-shell heterostructure and the heterojunction between the TiO2 core and the ZnS shell, TiO2-ZnS core-shell nanorods exhibited ethanol gas-sensing performance superior to that of pristine TiO2 nanorods. An optimal ZnO sacrificial shell layer thickness of approximately 60 nm was found to enable the synthesis of TiO2-ZnS composite nanorods with satisfactory gas-sensing performance through sulfidation. The results demonstrated that hydrothermally derived TiO2-ZnS core-shell composite nanorods with a sputter-deposited ZnO sacrificial shell layer are promising for applications in gas sensors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Shell Layer Thickness-Dependent Photocatalytic Activity of Sputtering Synthesized Hexagonally Structured ZnO-ZnS Composite Nanorods.Materials (Basel). 2018 Jan 7;11(1):87. doi: 10.3390/ma11010087. Materials (Basel). 2018. PMID: 29316671 Free PMC article.
-
Surface crystal feature-dependent photoactivity of ZnO-ZnS composite rods via hydrothermal sulfidation.RSC Adv. 2018 Jan 31;8(9):5063-5070. doi: 10.1039/c7ra13061a. eCollection 2018 Jan 24. RSC Adv. 2018. PMID: 35539554 Free PMC article.
-
Ultraintense UV emission from ZnO-sheathed ZnS nanorods.Sci Rep. 2017 Oct 12;7(1):13034. doi: 10.1038/s41598-017-13556-0. Sci Rep. 2017. PMID: 29026180 Free PMC article.
-
Microstructure-Dependent Visible-Light Driven Photoactivity of Sputtering-Assisted Synthesis of Sulfide-Based Visible-Light Sensitizer onto ZnO Nanorods.Materials (Basel). 2016 Dec 15;9(12):1014. doi: 10.3390/ma9121014. Materials (Basel). 2016. PMID: 28774134 Free PMC article.
-
Enhancement of the Sensing Performance of Devices based on Multistimuli-Responsive Hybrid Materials.ACS Appl Mater Interfaces. 2024 Nov 13;16(45):61408-61418. doi: 10.1021/acsami.3c08376. Epub 2023 Sep 13. ACS Appl Mater Interfaces. 2024. PMID: 37702609 Free PMC article. Review.
Cited by
-
Design of Nanoscaled Surface Morphology of TiO2-Ag2O Composite Nanorods through Sputtering Decoration Process and Their Low-Concentration NO2 Gas-Sensing Behaviors.Nanomaterials (Basel). 2019 Aug 11;9(8):1150. doi: 10.3390/nano9081150. Nanomaterials (Basel). 2019. PMID: 31405208 Free PMC article.
-
Enhancement of Acetone Gas-Sensing Responses of Tapered WO3 Nanorods through Sputtering Coating with a Thin SnO2 Coverage Layer.Nanomaterials (Basel). 2019 Jun 6;9(6):864. doi: 10.3390/nano9060864. Nanomaterials (Basel). 2019. PMID: 31174373 Free PMC article.
-
Crystal Design and Photoactivity of TiO2 Nanorod Template Decorated with Nanostructured Bi2S3 Visible Light Sensitizer.Int J Mol Sci. 2022 Oct 10;23(19):12024. doi: 10.3390/ijms231912024. Int J Mol Sci. 2022. PMID: 36233326 Free PMC article.
-
Design of Hydrothermally Derived Fe2O3 Rods with Enhanced Dual Functionality Via Sputtering Decoration of a Thin ZnO Coverage Layer.ACS Omega. 2020 Jun 22;5(26):16272-16283. doi: 10.1021/acsomega.0c02107. eCollection 2020 Jul 7. ACS Omega. 2020. PMID: 32656450 Free PMC article.
References
LinkOut - more resources
Full Text Sources

