First-principles study on the electrical and thermal properties of the semiconducting Sc3(CN)F2 MXene
- PMID: 35539724
- PMCID: PMC9081388
- DOI: 10.1039/c8ra03424a
First-principles study on the electrical and thermal properties of the semiconducting Sc3(CN)F2 MXene
Abstract
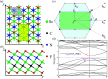
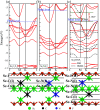
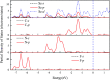
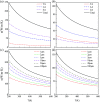
The two-dimensional materials MXenes have recently attracted interest for their excellent performance from diverse perspectives indicated by experiments and theoretical calculations. For the application of MXenes in electronic devices, the exploration of semiconducting MXenes arouses particular interest. In this work, despite the metallic properties of Sc3C2F2 and Sc3N2F2, we find that Sc3(CN)F2 is a semiconductor with an indirect band gap of 1.18 eV, which is an expansion of the semiconducting family members of MXene. Using first-principles calculations, the electrical and thermal properties of the semiconducting Sc3(CN)F2 MXene are studied. The electron mobilities are determined to possess strong anisotropy, while the hole mobilities show isotropy, i.e. 1.348 × 103 cm2 V-1 s-1 along x, 0.319 × 103 cm2 V-1 s-1 along the y directions for electron mobilities, and 0.517 × 103 cm2 V-1 s-1 along x, 0.540 × 103 cm2 V-1 s-1 along the y directions for hole mobilities. The room-temperature thermal conductivity along the Γ → M direction is determined to be 123-283 W m-1 K-1 with a flake length of 1-100 μm. Besides, Sc3(CN)F2 presents a relatively high specific heat of 547 J kg-1 K-1 and a low thermal expansion coefficient of 8.703 × 10-6 K-1. Our findings suggest that the Sc3(CN)F2 MXene might be a candidate material in the design and application of 2D nanoelectronic devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Promising electron mobility and high thermal conductivity in Sc2CT2 (T = F, OH) MXenes.Nanoscale. 2016 Mar 21;8(11):6110-7. doi: 10.1039/c5nr08639f. Nanoscale. 2016. PMID: 26932122
-
The thermal and electrical properties of the promising semiconductor MXene Hf2CO2.Sci Rep. 2016 Jun 15;6:27971. doi: 10.1038/srep27971. Sci Rep. 2016. PMID: 27302597 Free PMC article.
-
Two-dimensional semiconducting Lu2CT2 (T = F, OH) MXene with low work function and high carrier mobility.Nanoscale. 2020 Feb 14;12(6):3795-3802. doi: 10.1039/c9nr10806h. Epub 2020 Jan 29. Nanoscale. 2020. PMID: 31994570
-
MXene in the lens of biomedical engineering: synthesis, applications and future outlook.Biomed Eng Online. 2021 Apr 1;20(1):33. doi: 10.1186/s12938-021-00873-9. Biomed Eng Online. 2021. PMID: 33794899 Free PMC article. Review.
-
Effective Surface Modification of 2D MXene toward Thermal Energy Conversion and Management.Small Methods. 2023 Aug;7(8):e2300077. doi: 10.1002/smtd.202300077. Epub 2023 Apr 17. Small Methods. 2023. PMID: 37069766 Review.
Cited by
-
MXene Contact Engineering for Printed Electronics.Adv Sci (Weinh). 2023 Jul;10(19):e2207174. doi: 10.1002/advs.202207174. Epub 2023 Apr 25. Adv Sci (Weinh). 2023. PMID: 37096843 Free PMC article. Review.
-
Emerging Trends and Recent Progress of MXene as a Promising 2D Material for Point of Care (POC) Diagnostics.Diagnostics (Basel). 2023 Feb 12;13(4):697. doi: 10.3390/diagnostics13040697. Diagnostics (Basel). 2023. PMID: 36832187 Free PMC article. Review.
-
MXene-Embedded Electrospun Polymeric Nanofibers for Biomedical Applications: Recent Advances.Micromachines (Basel). 2023 Jul 23;14(7):1477. doi: 10.3390/mi14071477. Micromachines (Basel). 2023. PMID: 37512788 Free PMC article. Review.
-
First-principles study of magnetism in some novel MXene materials.RSC Adv. 2020 Dec 16;10(72):44430-44436. doi: 10.1039/d0ra03643a. eCollection 2020 Dec 9. RSC Adv. 2020. PMID: 35517132 Free PMC article.
References
-
- Hong Ng V. M. Huang H. Zhou K. Lee P. S. Que W. Xu J. Z. Kong L. B. J. Mater. Chem. A. 2017;5:3039–3068. doi: 10.1039/C6TA06772G. - DOI
-
- Khazaei M. Ranjbar A. Arai M. Sasaki T. Yunoki S. J. Mater. Chem. C. 2017;5:2488–2503. doi: 10.1039/C7TC00140A. - DOI
-
- Anasori B. Lukatskaya M. R. Gogotsi Y. Nat. Rev. Mater. 2017;2:16098. doi: 10.1038/natrevmats.2016.98. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

