Synthesis, spectroscopic, thermal, antimicrobial and electrochemical characterization of some novel Ru(iii), Pt(iv) and Ir(iii) complexes of pipemidic acid
- PMID: 35539728
- PMCID: PMC9081379
- DOI: 10.1039/c8ra03879a
Synthesis, spectroscopic, thermal, antimicrobial and electrochemical characterization of some novel Ru(iii), Pt(iv) and Ir(iii) complexes of pipemidic acid
Abstract
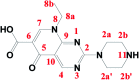
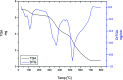
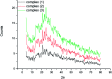
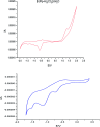
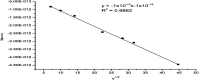
Three new solid complexes of pipemidic acid (Pip-H) with Ru3+, Pt4+ and Ir3+ were synthesized and characterized. Pipemidic acid acts as a uni-dentate chelator through the nitrogen atom of the -NH piperazyl ring. The spectroscopic data revealed that the general formulas of Pip-H complexes are [M(L) n (Cl) x ]·yH2O ((1) M = Ru3+, L: Pip-H, n = 3, x = 3, y = 6; (2) M = Pt4+, L: Pip-NH4, n = 2, x = 4, y = 0 and (3) M = Ir3+, L: Pip-H, n = 3, x = 3, y = 6). The number of water molecules with their locations inside or outside the coordination sphere were assigned via thermal analyses (TG, DTG). The DTG curves refer to 2-3 thermal decomposition steps where the first decomposition step at a lower temperature corresponds to the loss of uncoordinated water molecules followed by the decomposition of Pip-H molecules at higher temperatures. Thermodynamic parameters (E*, ΔS*, ΔH* and ΔG*) were calculated from the TG curves using Coats-Redfern and Horowitz-Metzeger non-isothermal models. X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) techniques were carefully used to assign properly the particle sizes of the prepared Pip-H complexes. The biological enhancement of Pip-H complexes rather than free chelate were assessed in vitro against four kinds of bacteria G(+) (Staphylococcus epidermidis and Staphylococcus aureus) and G(-) (Klebsiella and Escherichia coli) as well as against the human breast cancer (MCF-7) tumor cell line.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Fonseca I. Martinex-Carrera S. Garcia-Blanco S. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1986;42:1618–1621. doi: 10.1107/S0108270186091266. - DOI
-
- Toscano M. A. Serrao A. Ventimiglia B. Colosi V. Pennisi M. Morgana R. Bevilacqua T. Miner. Urol. 1982;34:257–260. - PubMed
-
- Babić S. Horvat A. J. M. Pavlović D. M. Kaštelan-Macan M. Trends Anal. Chem. 2007;26:1043–1061. doi: 10.1016/j.trac.2007.09.004. - DOI
-
- Efthimiadou E. K. Sanakis Y. Katsaros N. Karaliota A. Psomas G. Polyhedron. 2007;26:1148–1158. doi: 10.1016/j.poly.2006.10.017. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

