Efficient access to amides of the carborane carboxylic acid [1-(COOH)-CB11H11]
- PMID: 35539747
- PMCID: PMC9081092
- DOI: 10.1039/c8ra03067g
Efficient access to amides of the carborane carboxylic acid [1-(COOH)-CB11H11]
Abstract
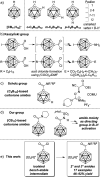
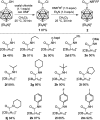
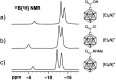
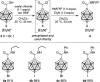
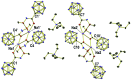
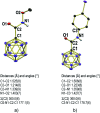
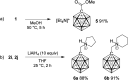
The preparation of the carborane acid chloride [1-(COCl)-CB11H11]- from the carboxylic acid [1-(COOH)-CB11H11]- is reported. This acid chloride exhibits remarkable inertness towards moisture and can be stored under ambient conditions for several months. Reaction with amines affords secondary and tertiary carborane amides [1-(CONR1R2)-CB11H11]- in moderate to high yields under mild conditions. Two of the amide products were characterized by X-ray crystallography in addition to spectroscopic analysis. Preliminary studies show that the amides can be reduced to the corresponding amines and that the acid chloride has the potential to serve as a starting material for carborane ester formation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Grimes R. N., Carboranes, Elsevier, Amsterdam, 3rd edn, 2016
- Hosmane N. S., Boron Science: New Technologies and Applications, Taylor & Francis/CRC, Boca Raton, 2011
-
- Douvris C. Michl J. Chem. Rev. 2013;113:PR179–PR233. doi: 10.1021/cr400059k. - DOI - PubMed
- Knapp C., Weakly Coordinating Anions: Halogenated Borates and Dodecaborates in Comprehensive Inorganic Chemistry II, Elsevier, Amsterdam, 2013, vol. 1, pp. 651–679
- Grimes R. N. Dalton Trans. 2015;44:5939–5956. doi: 10.1039/C5DT00231A. - DOI - PubMed
- Poater J. Solà M. Teixidor F. Chem.–Eur. J. 2016;22:7437–7443. doi: 10.1002/chem.201600510. - DOI - PubMed
- Melichar P. Hnyk D. Fanfrlík J. Phys. Chem. Chem. Phys. 2018;20:4666–4675. doi: 10.1039/C7CP07422K. - DOI - PubMed
- Axtell J. C. Saleh L. M. A. Qian E. A. Wixtrom A. I. Spokoyny A. M. Inorg. Chem. 2018;57:2333–2350. doi: 10.1021/acs.inorgchem.7b02912. - DOI - PMC - PubMed
-
- Finze M. Sprenger J. A. P. Schaack B. B. Dalton Trans. 2010;39:2708–2716. doi: 10.1039/B922720B. - DOI - PubMed
- Spokoyny A. M. Machan C. W. Clingerman D. J. Rosen M. S. Wiester M. J. Kennedy R. D. Stern C. L. Sarjeant A. A. Mirkin C. A. Nat. Chem. 2011;3:590–596. doi: 10.1038/nchem.1088. - DOI - PubMed
- Yao Z.-J. Jin G.-X. Coord. Chem. Rev. 2013;257:2522–2535. doi: 10.1016/j.ccr.2013.02.004. - DOI
- El-Hellani A. Lavallo V. Angew. Chem., Int. Ed. 2014;53:4489–4493. doi: 10.1002/anie.201402445. - DOI - PubMed
- Asay M. J. Fisher S. P. Lee S. E. Tham F. S. Borchardt D. Lavallo V. Chem. Commun. 2015;51:5359–5362. doi: 10.1039/C4CC08267B. - DOI - PubMed
- Riley L. E. Chan A. P. Y. Taylor J. Man W. Y. Ellis D. Rosair G. M. Welch A. J. Sivaev I. B. Dalton Trans. 2016;45:1127–1137. doi: 10.1039/C5DT03417E. - DOI - PubMed
- Estrada J. Lugo C. A. McArthur S. G. Lavallo V. Chem. Commun. 2016;52:1824–1826. doi: 10.1039/C5CC08377J. - DOI - PubMed
- Chan A. L. Estrada J. Kefalidis C. E. Lavallo V. Organometallics. 2016;35:3257–3260. doi: 10.1021/acs.organomet.6b00622. - DOI
- Fisher S. P. El-Hellani A. Tham F. S. Lavallo V. Dalton Trans. 2016;45:9762–9765. doi: 10.1039/C6DT00551A. - DOI - PubMed
- Holmes J. Pask C. M. Fox M. A. Willans C. E. Chem. Commun. 2016;52:6443–6446. doi: 10.1039/C6CC01650B. - DOI - PubMed
- Zhou Y.-P. Raoufmoghaddam S. Szilvási T. Driess M. Angew. Chem., Int. Ed. 2016;55:12868–12872. doi: 10.1002/anie.201606979. - DOI - PubMed
- Šembera F. Plutnar J. Higelin A. Janoušek Z. Císařová I. Michl J. Inorg. Chem. 2016;55:3797–3806. doi: 10.1021/acs.inorgchem.5b02678. - DOI - PubMed
- Coburger P. Schulz J. Klose J. Schwarze B. Sárosi M. B. Hey-Hawkins E. Inorg. Chem. 2017;56:292–304. doi: 10.1021/acs.inorgchem.6b02173. - DOI - PubMed
- Selg C. Neumann W. Lönnecke P. Hey-Hawkins E. Zeitler K. Chem.–Eur. J. 2017;23:7932–7937. doi: 10.1002/chem.201700209. - DOI - PubMed
- Estrada J. Lavallo V. Angew. Chem., Int. Ed. 2017;56:9906–9909. doi: 10.1002/anie.201705857. - DOI - PubMed
-
- Jude H. Disteldorf H. Fischer S. Wedge T. Hawkridge A. M. Arif A. M. Hawthorne M. F. Muddiman D. C. Stang P. J. J. Am. Chem. Soc. 2005;127:1231–12139. doi: 10.1021/ja053050i. - DOI - PubMed
- Huang S.-L. Weng L.-H. Jin G.-X. Dalton Trans. 2012;41:11657–11662. doi: 10.1039/C2DT30708A. - DOI - PubMed
- Kobr L. Zhao K. Shen Y. Shoemaker R. K. Rogers C. T. Michl J. Adv. Mater. 2013;25:443–448. doi: 10.1002/adma.201203294. - DOI - PubMed
- Kennedy R. D. Krungleviciute V. Clingerman D. J. Mondloch J. E. Peng Y. Wilmer C. E. Sarjeant A. A. Snurr R. Q. Hupp J. T. Yildirim T. Farha O. K. Mirkin C. A. Chem. Mater. 2013;25:3539–3543. doi: 10.1021/cm4020942. - DOI
- Han Y.-F. Jin G.-X. Acc. Chem. Res. 2014;47:3571–3579. doi: 10.1021/ar500335a. - DOI - PubMed
- Housecroft C. E. J. Organomet. Chem. 2015;798:218–228. doi: 10.1016/j.jorganchem.2015.04.047. - DOI
- Clingerman D. J. Morris W. Mondloch J. E. Kennedy R. D. Sarjieant A. A. Stern C. Hupp J. T. Farha O. K. Mirkin C. A. Chem. Commun. 2015;51:6521–6523. doi: 10.1039/C4CC09212K. - DOI - PubMed
- Rodríguez-Hermida S. Tsang M. Y. Vignatti C. Stylianou K. C. Guillerm V. Pérez-Carvajal J. Teixidor F. Viñas C. Choquesillo-Lazarte D. Verdugo-Escamilla C. Peral I. Juanhuix J. Verdaguer A. Imaz I. Maspoch D. Giner Planas J. Angew. Chem., Int. Ed. 2016;55:16049–16053. doi: 10.1002/anie.201609295. - DOI - PubMed
- Tsang M. Y. Rodríguez-Hermida S. Stylianou K. C. Tan F. Negi D. Teixidor F. Viñas C. Choquesillo-Lazarte D. Verdugo-Escamilla C. Guerrero M. Sort J. Juanhuix J. Maspoch D. Giner Planas J. Cryst. Growth Des. 2017;17:846–857. doi: 10.1021/acs.cgd.6b01682. - DOI
-
- Sivaev I. B. Bregadze V. V. Eur. J. Inorg. Chem. 2009:1433–1450. doi: 10.1002/ejic.200900003. - DOI
- Issa F. Kassiou M. Rendina L. M. Chem. Rev. 2011;111:5701–5722. doi: 10.1021/cr2000866. - DOI - PubMed
- Scholz M. H-Hawkins E. Chem. Rev. 2011;111:7035–7062. doi: 10.1021/cr200038x. - DOI - PubMed
- Gabel D. Pure Appl. Chem. 2015;87:173–179.
- Leśnikowski Z. J. J. Med. Chem. 2016;59:7738–7758. doi: 10.1021/acs.jmedchem.5b01932. - DOI - PubMed
LinkOut - more resources
Full Text Sources

