Extraction of isoflavones from Puerariae lobata using subcritical water
- PMID: 35539757
- PMCID: PMC9081427
- DOI: 10.1039/c8ra02653j
Extraction of isoflavones from Puerariae lobata using subcritical water
Abstract
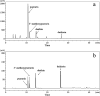
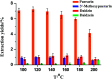
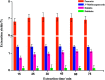
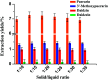
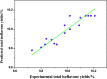
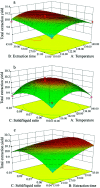
As an alternative to organic solvents, subcritical water was employed for the first time as an effective solvent for the extraction of isoflavones from Puerariae lobata. Optimum experimental conditions for the extraction of the four main isoflavones were established by single factor experiments, and the optimum experimental conditions for total isoflavone extraction were established further by response surface methodology. With an extraction time of 45 min and a liquid/solid ratio of 1 : 20, the extraction yields of puerarin, 3'-methoxypuerarin, and daidzin reached maxima at extraction temperatures of 120 °C, 140 °C and 200 °C, respectively. Moreover, puerarin, 3'-methoxypuerarin and daidzin were degraded and produced various byproducts due to hydrothermal reactions at higher temperatures. The maximum extraction yields of the total isoflavones were obtained by response surface methodology (extraction time of 45 min, solid/liquid ratio of 1 : 15 and extraction temperature of 120 °C). Compared to conventional solvents, subcritical water utilized less solvent and required a shorter extraction time.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures
Similar articles
-
Optimisation of ultrasound-assisted extraction of puerarin and total isoflavones from Puerariae Lobatae Radix (Pueraria lobata (Wild.) Ohwi) with response surface methodology.Phytochem Anal. 2012 Sep-Oct;23(5):513-9. doi: 10.1002/pca.2349. Epub 2012 Jan 18. Phytochem Anal. 2012. PMID: 22259187
-
Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity.Molecules. 2020 Jan 15;25(2):344. doi: 10.3390/molecules25020344. Molecules. 2020. PMID: 31952126 Free PMC article.
-
Optimization and kinetics modeling of okara isoflavones extraction using subcritical water.Food Chem. 2019 Oct 15;295:613-621. doi: 10.1016/j.foodchem.2019.05.129. Epub 2019 May 20. Food Chem. 2019. PMID: 31174803
-
Seasonal variations in the isoflavonoids of radix Puerariae.Phytochem Anal. 2007 May-Jun;18(3):245-50. doi: 10.1002/pca.978. Phytochem Anal. 2007. PMID: 17500368
-
Analysis of isoflavone daidzein in Puerariae radix with micelle-mediated extraction and preconcentration.J Agric Food Chem. 2005 Feb 9;53(3):518-23. doi: 10.1021/jf048545q. J Agric Food Chem. 2005. PMID: 15686396
Cited by
-
Comparison of the Extraction Efficiency of Isoflavone Compounds from Puerariae lobatae by Ionic Liquids with 11 Anions and 8 Imidazolium-Based Cations.ACS Omega. 2020 Apr 9;5(15):8962-8971. doi: 10.1021/acsomega.0c00724. eCollection 2020 Apr 21. ACS Omega. 2020. PMID: 32337460 Free PMC article.
-
Subcritical Water Extraction of Natural Products.Molecules. 2021 Jun 30;26(13):4004. doi: 10.3390/molecules26134004. Molecules. 2021. PMID: 34209151 Free PMC article. Review.
-
Green Approaches to Sample Preparation Based on Extraction Techniques.Molecules. 2020 Apr 9;25(7):1719. doi: 10.3390/molecules25071719. Molecules. 2020. PMID: 32283595 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

