Xyloplains A-F, six new guaiane-type sesquiterpenoid dimers from Xylopia vielana
- PMID: 35539790
- PMCID: PMC9082531
- DOI: 10.1039/c8ra04356f
Xyloplains A-F, six new guaiane-type sesquiterpenoid dimers from Xylopia vielana
Abstract
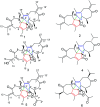
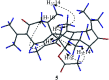
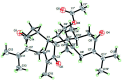
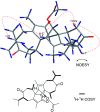
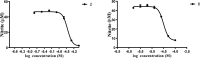
Six new guaiane dimers, xyloplains A-F (1-6), with connecting patterns through two direct C-C bonds (C-1 to C-3', C-2 to C-1'), were isolated from the roots of Xylopia vielana. Their structures were elucidated clearly using extensive analysis of 1D NMR and 2D NMR, combined with Cu-Kα X-ray diffraction and circular dichroism (CD) experiments. In additon, all of the isolates were tested for anti-inflammatory activity by measuring the amount of nitric oxide produced. To our delight, compounds 2 and 6 exhibited moderate inhibitory activity against the production of nitric oxide with IC50 value of 34.5 and 31.1 μM, respectively, in RAW264.7 cells stimulated by LPS.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Vielopsides A-E, five new guaiane-type sesquiterpenoid dimers from Xylopia vielana.Fitoterapia. 2018 Oct;130:43-47. doi: 10.1016/j.fitote.2018.07.020. Epub 2018 Aug 1. Fitoterapia. 2018. PMID: 30076886
-
Xylopsides A-D, four rare guaiane dimers with two unique bridged pentacyclic skeletons from Xylopia vielana.Org Biomol Chem. 2018 Sep 26;16(37):8408-8412. doi: 10.1039/c8ob01689e. Org Biomol Chem. 2018. PMID: 30221279
-
Vieloplains A-G, seven new guaiane-type sesquiterpenoid dimers from Xylopia vielana.Bioorg Chem. 2019 Jul;88:102891. doi: 10.1016/j.bioorg.2019.03.065. Epub 2019 Apr 10. Bioorg Chem. 2019. PMID: 30999244
-
Xylopidimers A-E, Five New Guaiane Dimers with Various Carbon Skeletons from the Roots of Xylopia vielana.ACS Omega. 2019 Jan 25;4(1):2047-2052. doi: 10.1021/acsomega.8b00828. eCollection 2019 Jan 31. ACS Omega. 2019. PMID: 31459455 Free PMC article.
-
Chlomultiols A-L, sesquiterpenoids from Chloranthus multistachys and their anti-inflammatory activities.Phytochemistry. 2022 Jan;193:113001. doi: 10.1016/j.phytochem.2021.113001. Epub 2021 Nov 8. Phytochemistry. 2022. PMID: 34763221 Review.
References
-
- Martins D. Osshiro E. Roque N. F. Marks V. Gottlieb H. E. Phytochemistry. 1998;48:677–680. doi: 10.1016/S0031-9422(97)01048-0. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

