A MOF-based carrier for in situ dopamine delivery
- PMID: 35539814
- PMCID: PMC9082660
- DOI: 10.1039/c8ra04969f
A MOF-based carrier for in situ dopamine delivery
Abstract
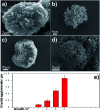
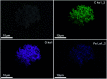
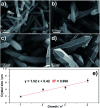
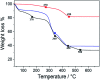
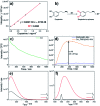
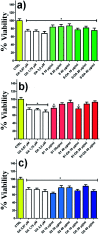
MIL-88A (Fe) MOF crystals were nucleated and grown around a polymer core containing superparamagnetic nanoparticles to assemble a new class of biocompatible particles for magnetophoretic drug delivery of dopamine. The carrier enabled efficient targeted release, dopamine protection from oxidative damage, long-term delivery and improved drug delivery cost-efficiency. After loading, dopamine was stable within the carrier and did not undergo oxidation. Drug release monitoring via spectrofluorimetry revealed a shorter burst effect and higher release efficiency than silica based carriers. The in vitro cytotoxicity at different MOF concentrations and sizes was assessed using PC12 cells as the neuronal cell model. The drug was directly uptaken into the PC12 cells avoiding possible side effects due to oxidation occurring in the extracellular environment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Metal Organic Framework (MOF) Particles as Potential Bacteria-Mimicking Delivery Systems for Infectious Diseases: Characterization and Cellular Internalization in Alveolar Macrophages.Pharm Res. 2019 Feb 21;36(4):53. doi: 10.1007/s11095-019-2589-4. Pharm Res. 2019. PMID: 30790066
-
Heterostructured Ag@MOF-801/MIL-88A(Fe) Nanocomposite as a Biocompatible Photocatalyst for Degradation of Reactive Black 5 under Visible Light.Inorg Chem. 2023 Oct 30;62(43):17818-17829. doi: 10.1021/acs.inorgchem.3c02616. Epub 2023 Oct 19. Inorg Chem. 2023. PMID: 37856158
-
Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery.Nanoscale. 2017 Jun 8;9(22):7454-7463. doi: 10.1039/c6nr07593b. Nanoscale. 2017. PMID: 28530283
-
Functionalized Calcium Carbonate-Based Microparticles as a Versatile Tool for Targeted Drug Delivery and Cancer Treatment.Pharmaceutics. 2024 May 13;16(5):653. doi: 10.3390/pharmaceutics16050653. Pharmaceutics. 2024. PMID: 38794315 Free PMC article. Review.
-
Recent Advances in Fe-MOF Compositions for Biomedical Applications.Curr Med Chem. 2021;28(30):6179-6198. doi: 10.2174/0929867328666210511014129. Curr Med Chem. 2021. PMID: 33992053 Review.
Cited by
-
Metal-Organic Framework (MOF)-Based Biomaterials for Tissue Engineering and Regenerative Medicine.Front Bioeng Biotechnol. 2021 Mar 11;9:603608. doi: 10.3389/fbioe.2021.603608. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33777907 Free PMC article. Review.
-
Modulating the stacking modes of nanosized metal-organic frameworks by morphology engineering for isomer separation.Chem Sci. 2021 Feb 3;12(11):4104-4110. doi: 10.1039/d0sc06747d. Chem Sci. 2021. PMID: 34163681 Free PMC article.
-
Biomedical Applications of Metal-Organic Frameworks Revisited.Ind Eng Chem Res. 2025 Jan 14;64(4):1907-1932. doi: 10.1021/acs.iecr.4c03698. eCollection 2025 Jan 29. Ind Eng Chem Res. 2025. PMID: 39906289 Free PMC article. Review.
-
Inhibitory Effect of Curcumin-Inspired Derivatives on Tyrosinase Activity and Melanogenesis.Molecules. 2022 Nov 16;27(22):7942. doi: 10.3390/molecules27227942. Molecules. 2022. PMID: 36432043 Free PMC article.
-
BioMOF-Based Anti-Cancer Drug Delivery Systems.Nanomaterials (Basel). 2023 Mar 6;13(5):953. doi: 10.3390/nano13050953. Nanomaterials (Basel). 2023. PMID: 36903831 Free PMC article. Review.
References
-
- Kageyama T. Nakamura M. Matsuo A. Yamasaki Y. Takakura Y. Hashida M. Kanai Y. Naito M. Tsuruo T. Minato N. Shimohama S. Brain Res. 2000;8:79115–79121. - PubMed
-
- Di Gioia S. Trapani A. Mandracchia D. De Giglio E. Cometa S. Mangini V. Arnesano F. Belgiovine G. Castellani S. Pace L. Lavecchia M. A. Trapani G. Conese M. Puglisi G. Cassano T. Eur. J. Pharm. Biopharm. 2015;9:4180–4193. - PubMed
LinkOut - more resources
Full Text Sources

