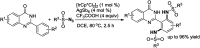Iridium-catalysed direct sulfamidation of quinazolinones
- PMID: 35539849
- PMCID: PMC9078565
- DOI: 10.1039/c8ra00524a
Iridium-catalysed direct sulfamidation of quinazolinones
Abstract
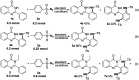
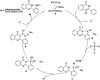
Ir-catalysed direct sulfamidation of quinazolinones has been achieved. A series of ortho-diamided quinazolinones were obtained in up to 96% yields. This transformation could proceed smoothly with a low catalyst loading under mild conditions with nitrogen released as the sole byproduct. This approach potentially provides an environmentally benign sulfamidation process for atom/step economic syntheses of useful pharmaceutical molecules or important building blocks.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Iridium-Catalyzed Direct ortho-C-H Amidation of Benzaldehydes through N-Sulfonyl Imines as Mask.Org Lett. 2016 Oct 7;18(19):4924-4927. doi: 10.1021/acs.orglett.6b02406. Epub 2016 Sep 20. Org Lett. 2016. PMID: 27644157
-
Iridium-Catalyzed Direct C-H Sulfamidation of Aryl Nitrones with Sulfonyl Azides at Room Temperature.J Org Chem. 2015 Aug 7;80(15):7333-9. doi: 10.1021/acs.joc.5b01377. Epub 2015 Jul 21. J Org Chem. 2015. PMID: 26182385
-
Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: a journey for the mild and versatile C-H amination.Acc Chem Res. 2015 Apr 21;48(4):1040-52. doi: 10.1021/acs.accounts.5b00020. Epub 2015 Mar 30. Acc Chem Res. 2015. PMID: 25821998
-
Recent advances and applications of iridium-catalysed asymmetric allylic substitution.Org Biomol Chem. 2012 Apr 28;10(16):3147-63. doi: 10.1039/c2ob07086c. Epub 2012 Mar 12. Org Biomol Chem. 2012. PMID: 22407450 Review.
-
A Single-Atom Iridium Heterogeneous Catalyst in Oxygen Reduction Reaction.Angew Chem Int Ed Engl. 2019 Jul 8;58(28):9640-9645. doi: 10.1002/anie.201905241. Epub 2019 Jun 18. Angew Chem Int Ed Engl. 2019. PMID: 31120620 Review.
References
-
-
For selected reviews see:
- Doyle M. P. Goldberg K. I. Acc. Chem. Res. 2012;45:777. doi: 10.1021/ar300096z. - DOI - PubMed
- Bruckl T. Baxter R. D. Ishihara Y. Baran P. S. Acc. Chem. Res. 2012;45:826. doi: 10.1021/ar200194b. - DOI - PMC - PubMed
- Arockiam P. B. Bruneau C. Dixneuf P. H. Chem. Rev. 2012;112:5879. doi: 10.1021/cr300153j. - DOI - PubMed
- Sun C.-L. Li B.-J. Shi Z.-J. Chem. Rev. 2011;111:1293. doi: 10.1021/cr100198w. - DOI - PubMed
- Lyons T. W. Sanford M. S. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. - DOI - PMC - PubMed
- Yamaguchi J. Yamaguchi A. D. Itami K. Angew. Chem., Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666. - DOI - PubMed
- Colby D. A. Bergman R. G. Ellman J. A. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. - DOI - PMC - PubMed
- Wencel-Delord J. Drcge T. Liu F. Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/C1CS15083A. - DOI - PubMed
- Satoh T. Miua M. Chem.–Eur. J. 2010;16:11212. doi: 10.1002/chem.201001363. - DOI - PubMed
- Song G. Y. Wang F. Li X. Chem. Soc. Rev. 2012;41:3651. doi: 10.1039/C2CS15281A. - DOI - PubMed
- Ackermann L. Pospech J. Org. Lett. 2011;13:4153. doi: 10.1021/ol201563r. - DOI - PubMed
- Ueyama T. Mochida S. Fukutani T. Hirano K. Satoh T. Miura M. Org. Lett. 2011;13:706. doi: 10.1021/ol102942w. - DOI - PubMed
- Weissman H. Song X. Milstein D. J. Am. Chem. Soc. 2001;123:337. doi: 10.1021/ja003361n. - DOI - PubMed
- Wu J. Cui X. Mi X. Li Y. Wu Y. Chem. Commun. 2010;46:6771. doi: 10.1039/C0CC01448F. - DOI - PubMed
- Ackermann L. Wang L. Lygin A. V. Chem. Sci. 2012;3:177. doi: 10.1039/C1SC00619C. - DOI
-
-
- Yang W. Wang J. Wei Z. Zhang Q. Xu X. J. Org. Chem. 2016;81:1675. doi: 10.1021/acs.joc.5b02903. - DOI - PubMed
- Walker S. E. Jordan-Hore J. A. Johnson D. G. Macgregor S. A. Lee A. L. Angew. Chem., Int. Ed. 2014;53:13876. doi: 10.1002/anie.201408054. - DOI - PMC - PubMed
- Honraedt A. Le Callonnec F. Le Grognec E. Fernandez V. Felpin F.-X. J. Org. Chem. 2013;78:4604. doi: 10.1021/jo4004426. - DOI - PubMed
- Zhou T. Li L. Li B. Song H. Wang B. Org. Lett. 2015;17:4204. doi: 10.1021/acs.orglett.5b01974. - DOI - PubMed
- Fujiwara Y. Domingo V. Seiple I. B. Gianatassio R. Del Bel M. D. Baran P. S. J. Am. Chem. Soc. 2011;133:3292. doi: 10.1021/ja111152z. - DOI - PMC - PubMed
- Shaaban S. Jolit A. Petkova D. Maulide N. Chem. Commun. 2015;51:13902. doi: 10.1039/C5CC03580E. - DOI - PubMed
- Jardim G. A. Bower J. F. Da S. J. E. Org. Lett. 2016;18:4454. doi: 10.1021/acs.orglett.6b01586. - DOI - PubMed
- Li B.-J. Shi Z.-J. Chem. Soc. Rev. 2012;41:5588. doi: 10.1039/C2CS35096C. - DOI - PubMed
- Kuhl N. Schroder N. Glorius F. Adv. Synth. Catal. 2014;356:1443. doi: 10.1002/adsc.201400197. - DOI
- Mo J. Wang L. Liu Y. Cui X. Synthesis. 2015;47:439. doi: 10.1055/s-0034-1379890. - DOI
-
- Ackermann L. Vicente R. Kapdi A. R. Angew. Chem., Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996. - DOI - PubMed
- Alberico D. Scott M. E. Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. - DOI - PubMed
- Lyons T. W. Sanford M. S. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. - DOI - PMC - PubMed
- Giri R. Shi B.-F. Engle K. M. Maugel N. Yu J.-Q. Chem. Soc. Rev. 2009;38:3242. doi: 10.1039/B816707A. - DOI - PubMed
- McMurray L. O'Hara F. Gaunt M. J. Chem. Soc. Rev. 2011;40:1885. doi: 10.1039/C1CS15013H. - DOI - PubMed
- Battiste M. A. Pelphrey P. M. Wright D. L. Chem.–Eur. J. 2006;12:3438. doi: 10.1002/chem.200501083. - DOI - PubMed
- Butenschön H. Angew. Chem., Int. Ed. 2008;47:5287. doi: 10.1002/anie.200801738. - DOI - PubMed
- Ylijoki K. E. O. Stryker J. M. Chem. Rev. 2013;113:2244. doi: 10.1021/cr300087g. - DOI - PubMed
- Harmata M. Chem. Commun. 2010;46:8886. doi: 10.1039/C0CC03620J. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources