Exploring the trans-membrane dynamic mechanisms of single polyamidoamine nano-drugs via a "force tracing" technique
- PMID: 35539864
- PMCID: PMC9078602
- DOI: 10.1039/c8ra00134k
Exploring the trans-membrane dynamic mechanisms of single polyamidoamine nano-drugs via a "force tracing" technique
Abstract
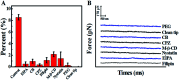
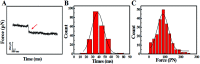
Considerable technological success has been achieved in the drug delivery of nano-drugs for chemotherapy, but the main obstacles in understanding the drug delivery dynamic mechanisms for nano-drug applications stem from technical limitations. In this paper, we explored the trans-membrane dynamic processes of polyamidoamine nano-drugs via a "force tracing" technique.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Studying the dynamic mechanism of transporting a single drug carrier-polyamidoamine dendrimer through cell membranes by force tracing.Nanoscale. 2016 Oct 27;8(42):18027-18031. doi: 10.1039/c6nr05838h. Nanoscale. 2016. PMID: 27734053
-
Dynamics of delivering aptamer targeted nano-drugs into cells.J Mater Chem B. 2021 Jan 28;9(4):952-957. doi: 10.1039/d0tb02527e. Epub 2021 Jan 13. J Mater Chem B. 2021. PMID: 33437980
-
Exploring the role of polymeric conjugates toward anti-cancer drug delivery: Current trends and future projections.Int J Pharm. 2018 Sep 5;548(1):500-514. doi: 10.1016/j.ijpharm.2018.06.060. Epub 2018 Jun 28. Int J Pharm. 2018. PMID: 29964172 Review.
-
Manipulating dynamic tumor vessel permeability to enhance polymeric micelle accumulation.J Control Release. 2021 Jan 10;329:63-75. doi: 10.1016/j.jconrel.2020.11.063. Epub 2020 Dec 2. J Control Release. 2021. PMID: 33278478
-
Inorganic Nano-Targeted Drugs Delivery System and Its Application of Platinum-Based Anticancer Drugs.J Nanosci Nanotechnol. 2017 Jan;17(1):1-17. doi: 10.1166/jnn.2017.12932. J Nanosci Nanotechnol. 2017. PMID: 29616785 Review.
Cited by
-
Big Data Analysis of Manufacturing and Preclinical Studies of Nanodrug-Targeted Delivery Systems: A Literature Review.Biomed Res Int. 2022 Jul 30;2022:1231446. doi: 10.1155/2022/1231446. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2023 Jun 21;2023:9759424. doi: 10.1155/2023/9759424. PMID: 35941977 Free PMC article. Retracted. Review.
-
Studying structure and functions of cell membranes by single molecule biophysical techniques.Biophys Rep. 2021 Oct 31;7(5):384-398. doi: 10.52601/bpr.2021.210018. Biophys Rep. 2021. PMID: 37288104 Free PMC article.
-
Size-Independent Transmembrane Transporting of Single Tetrahedral DNA Nanostructures.Glob Chall. 2019 Nov 20;4(3):1900075. doi: 10.1002/gch2.201900075. eCollection 2020 Mar. Glob Chall. 2019. PMID: 32140254 Free PMC article.
References
LinkOut - more resources
Full Text Sources

