5-Selenocyanato and 5-trifluoromethanesulfonyl derivatives of 2'-deoxyuridine: synthesis, radiation and computational chemistry as well as cytotoxicity
- PMID: 35539961
- PMCID: PMC9080949
- DOI: 10.1039/c8ra03172j
5-Selenocyanato and 5-trifluoromethanesulfonyl derivatives of 2'-deoxyuridine: synthesis, radiation and computational chemistry as well as cytotoxicity
Abstract
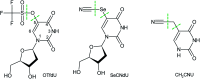
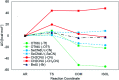
5-Selenocyanato-2'-deoxyuridine (SeCNdU) and 5-trifluoromethanesulfonyl-2'-deoxyuridine (OTfdU) have been synthesized and their structures have been confirmed with NMR and MS methods. Both compounds undergo dissociative electron attachment (DEA) when irradiated with X-rays in an aqueous solution containing a hydroxyl radical scavenger. The DEA yield of SeCNdU significantly exceeds that of 5-bromo-2'-deoxyuridine (BrdU), remaining in good agreement with the computationally revealed profile of electron-induced degradation. The radiolysis products indicate, in line with theoretical predictions, Se-CN bond dissociation as the main reaction channel. On the other hand, the DEA yield for OTfdU is slightly lower than the degradation yield measured for BrdU, despite the fact that the calculated driving force for the electron-induced OTfdU dissociation substantially overpasses the thermodynamic stimulus for BrdU degradation. Moreover, the calculated DEA profile suggests that the electron attachment induced formation of 5-hydroxy-2'-deoxyuridine (OHdU) from OTfdU, while 2'-deoxyuridine (dU) is mainly observed experimentally. We explained this discrepancy in terms of the increased acidity of OTfdU resulting in efficient deprotonation of the N3 atom, which brings about the domination of the OTfdU(N3-H)- anion in the equilibrium mixture. As a consequence, electron addition chiefly leads to the radical dianion, OTfdU(N3-H)˙2-, which easily protonates at the C5 site. As a result, the C5-O rather than O-S bond undergoes dissociation, leading to dU, observed experimentally. A negligible cytotoxicity of the studied compounds toward the MCF-7 cell line at the concentrations used for cell labelling calls for further studies aiming at the clinical use of the proposed derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Why Does the Type of Halogen Atom Matter for the Radiosensitizing Properties of 5-Halogen Substituted 4-Thio-2'-Deoxyuridines?Molecules. 2019 Aug 2;24(15):2819. doi: 10.3390/molecules24152819. Molecules. 2019. PMID: 31382376 Free PMC article.
-
Electron-Induced Dissociation of the Potential Radiosensitizer 5-Selenocyanato-2'-deoxyuridine.J Phys Chem B. 2019 Feb 14;123(6):1274-1282. doi: 10.1021/acs.jpcb.8b11523. Epub 2019 Feb 5. J Phys Chem B. 2019. PMID: 30657689
-
5-Thiocyanato-2'-deoxyuridine as a possible radiosensitizer: electron-induced formation of uracil-C5-thiyl radical and its dimerization.Phys Chem Chem Phys. 2015 Jul 14;17(26):16907-16. doi: 10.1039/c5cp02081f. Phys Chem Chem Phys. 2015. PMID: 26059609 Free PMC article.
-
Pathways of the Dissociative Electron Attachment Observed in 5- and 6-Azidomethyluracil Nucleosides: Nitrogen (N2) Elimination vs Azide Anion (N3-) Elimination.J Phys Chem B. 2023 Feb 23;127(7):1563-1571. doi: 10.1021/acs.jpcb.2c08257. Epub 2023 Feb 13. J Phys Chem B. 2023. PMID: 36780335 Free PMC article.
-
Influence of Hypoxia on Radiosensitization of Cancer Cells by 5-Bromo-2'-deoxyuridine.Int J Mol Sci. 2022 Jan 27;23(3):1429. doi: 10.3390/ijms23031429. Int J Mol Sci. 2022. PMID: 35163354 Free PMC article.
Cited by
-
Why Does the Type of Halogen Atom Matter for the Radiosensitizing Properties of 5-Halogen Substituted 4-Thio-2'-Deoxyuridines?Molecules. 2019 Aug 2;24(15):2819. doi: 10.3390/molecules24152819. Molecules. 2019. PMID: 31382376 Free PMC article.
-
Dissociative Electron Attachment to 5-Iodo-4-thio-2'-deoxyuridine: A Potential Radiosensitizer of Hypoxic Cells.J Phys Chem Lett. 2023 Oct 12;14(40):8948-8955. doi: 10.1021/acs.jpclett.3c02219. Epub 2023 Sep 28. J Phys Chem Lett. 2023. PMID: 37769041 Free PMC article.
-
Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents.Int J Mol Sci. 2021 Jul 23;22(15):7879. doi: 10.3390/ijms22157879. Int J Mol Sci. 2021. PMID: 34360644 Free PMC article. Review.
-
Uracil-5-yl O-Sulfamate: An Illusive Radiosensitizer. Pitfalls in Modeling the Radiosensitizing Derivatives of Nucleobases.J Phys Chem B. 2020 Jul 9;124(27):5600-5613. doi: 10.1021/acs.jpcb.0c03844. Epub 2020 Jun 28. J Phys Chem B. 2020. PMID: 32539395 Free PMC article.
-
Substituted benzylamino-2'-deoxyadenosine a modified nucleoside with radiosensitizing properties.Sci Rep. 2025 May 20;15(1):17535. doi: 10.1038/s41598-025-99262-8. Sci Rep. 2025. PMID: 40394130 Free PMC article.
References
-
- Joiner M. and van der Kogel A., Basic Clinical Radiobiology, Hodder Arnold, London, UK, 4th edn, 2009
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

