Uptake and distribution characteristics of the novel fungicide pyraoxystrobin in cucumber plants
- PMID: 35539991
- PMCID: PMC9083277
- DOI: 10.1039/c8ra05140b
Uptake and distribution characteristics of the novel fungicide pyraoxystrobin in cucumber plants
Abstract
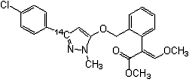
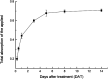
The uptake and distribution characteristics of a novel fungicide, pyraoxystrobin, labeled with 14C on its pyrazol ring, were investigated in cucumber (Cucumis sativus) seedlings. Foliar applied pyraoxystrobin rapidly penetrated the treated leaf and reached a maximum uptake of 68% after 5 d. The translocation of absorbed 14C in cucumber seedlings was both acropetal and basipetal. However, over 74% of the absorbed 14C-pyraoxystrobin remained in the treated leaves. The order of its distribution in the plant was as follows: treated leaf > stalk above the treated leaf > leaves above the treated leaf > stalk below the treated leaf > leaves below the treated leaf > cotyledon > root. Seedlings grown in soils containing bound residues (BR) of pyraoxystrobin revealed that the BRs were not easily absorbed or translocated by the plant. Soil type had a large effect on root uptake, with the highest uptake among the three tested soils from red clay.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Fate characterization of bound residues of 14C-Pyraoxystrobin in soils.Chemosphere. 2021 Jan;263:128023. doi: 10.1016/j.chemosphere.2020.128023. Epub 2020 Aug 18. Chemosphere. 2021. PMID: 33297046
-
Fate of a novel strobilurin fungicide pyraoxystrobin in flooded soil.Environ Sci Process Impacts. 2014 May;16(6):1495-500. doi: 10.1039/c3em00706e. Environ Sci Process Impacts. 2014. PMID: 24752609
-
Translocation and residue of 14C-benzene kresoxim-methyl in mature cucumber (Cucumis sativus L.).Sci Total Environ. 2021 Apr 20;766:144426. doi: 10.1016/j.scitotenv.2020.144426. Epub 2021 Jan 6. Sci Total Environ. 2021. PMID: 33421785
-
Adsorption and leaching of novel fungicide pyraoxystrobin on soils by 14C tracing method.Environ Monit Assess. 2018 Jan 19;190(2):86. doi: 10.1007/s10661-017-6458-5. Environ Monit Assess. 2018. PMID: 29349621
-
Absorption, translocation, and residue of 14C-ZJ0273 in oilseed rape.J Agric Food Chem. 2009 May 27;57(10):4398-402. doi: 10.1021/jf9001389. Epub 2009 Apr 17. J Agric Food Chem. 2009. PMID: 19371143
References
-
- Becker W. F. Jagow V. G. Anke T. Steglich W. Oudemansin, strobilurin A, strobilurin B and myxothiazol: New inhibitors of the bc1 segment of the respiratory chain with an e-β-methoxyacrylate system as common structural elements. FEBS Lett. 1981;132:329–333. doi: 10.1016/0014-5793(81)81190-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources

