Rhodopseudomonas palustris-based conversion of organic acids to hydrogen using plasmonic nanoparticles and near-infrared light
- PMID: 35540054
- PMCID: PMC9076380
- DOI: 10.1039/c9ra08747h
Rhodopseudomonas palustris-based conversion of organic acids to hydrogen using plasmonic nanoparticles and near-infrared light
Abstract
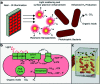
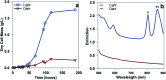
The simultaneous elimination of organic waste and the production of clean fuels will have an immense impact on both the society and the industrial manufacturing sector. The enhanced understanding of the interface between nanoparticles and photo-responsive bacteria will further advance the knowledge of their interactions with biological systems. Although literature shows the production of gases by photobacteria, herein, we demonstrated the integration of photonics, biology, and nanostructured plasmonic materials for hydrogen production with a lower greenhouse CO2 gas content at quantified light energy intensity and wavelength. Phototrophic purple non-sulfur bacteria were able to generate hydrogen as a byproduct of nitrogen fixation using the energy absorbed from visible and near-IR (NIR) light. This type of biological hydrogen production has suffered from low efficiency of converting light energy into hydrogen in part due to light sources that do not exploit the organisms' capacity for NIR absorption. We used NIR light sources and optically resonant gold-silica core-shell nanoparticles to increase the light utilization of the bacteria to convert waste organic acids such as acetic and maleic acids to hydrogen. The batch growth studies for the small cultures (40 mL) of Rhodopseudomonas palustris demonstrated >2.5-fold increase in hydrogen production when grown under an NIR source (167 ± 18 μmol H2) compared to that for a broad-band light source (60 ± 6 μmol H2) at equal light intensity (130 W m-2). The addition of the mPEG-coated optically resonant gold-silica core-shell nanoparticles in the solution further improved the hydrogen production from 167 ± 18 to 398 ± 108 μmol H2 at 130 W m-2. The average hydrogen production rate with the nanoparticles was 127 ± 35 μmol L-1 h-1 at 130 W m-2.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yilanci A. Dincer I. Ozturk H. K. Prog. Energy Combust. Sci. 2009;35:231–244. doi: 10.1016/j.pecs.2008.07.004. - DOI
-
- Padovani G. Vaičiulytė S. Carlozzi P. Fuel. 2016;166:203–210. doi: 10.1016/j.fuel.2015.10.124. - DOI
-
- Patsoura A. Kondarides D. I. Verykios X. E. Catal. Today. 2007;124:94–102. doi: 10.1016/j.cattod.2007.03.028. - DOI
-
- Zhou C. Lai C. Zhang C. Zeng G. Huang D. Cheng M. Hu L. Xiong W. Chen M. Wang J. Appl. Catal., B. 2018;238:6–18. doi: 10.1016/j.apcatb.2018.07.011. - DOI
-
- Zhou C. Xu P. Lai C. Zhang C. Zeng G. Huang D. Cheng M. Hu L. Xiong W. Wen X. Chem. Eng. J. 2019;359:186–196. doi: 10.1016/j.cej.2018.11.140. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

