Blast furnace slag-Mg(OH)2 cements activated by sodium carbonate
- PMID: 35540122
- PMCID: PMC9081583
- DOI: 10.1039/c8ra03717e
Blast furnace slag-Mg(OH)2 cements activated by sodium carbonate
Abstract
The structural evolution of a sodium carbonate activated slag cement blended with varying quantities of Mg(OH)2 was assessed. The main reaction products of these blended cements were a calcium-sodium aluminosilicate hydrate type gel, an Mg-Al layered double hydroxide with a hydrotalcite type structure, calcite, and a hydrous calcium aluminate phase (tentatively identified as a carbonate-containing AFm structure), in proportions which varied with Na2O/slag ratios. Particles of Mg(OH)2 do not chemically react within these cements. Instead, Mg(OH)2 acts as a filler accelerating the hardening of sodium carbonate activated slags. Although increased Mg(OH)2 replacement reduced the compressive strength of these cements, pastes with 50 wt% Mg(OH)2 still reached strengths of ∼21 MPa. The chemical and mechanical characteristics of sodium carbonate activated slag/Mg(OH)2 cements makes them a potentially suitable matrix for encapsulation of high loadings of Mg(OH)2-bearing wastes such as Magnox sludge.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




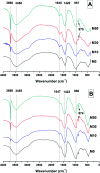













References
-
- Provis J. L. Bernal S. A. Annu. Rev. Mater. Res. 2014;44:299–327. doi: 10.1146/annurev-matsci-070813-113515. - DOI
-
- Shi C., Krivenko P. V. and Roy D., Alkali-Activated Cements and Concretes, Taylor & Francis, Abingdon, UK, 2006
-
- Bai Y. Collier N. C. Milestone N. B. Yang C. H. J. Nucl. Mater. 2011;413:183–192. doi: 10.1016/j.jnucmat.2011.04.011. - DOI
-
- Bernal S. A. Provis J. L. Myers R. J. San Nicolas R. van Deventer J. S. J. Mater. Struct. 2015;48:517–529. doi: 10.1617/s11527-014-0412-6. - DOI
-
- Bernal S. A. RILEM Tech. Lett. 2016;1:39–44. doi: 10.21809/rilemtechlett.2016.8. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

