Acyclic and cyclic imines and their metal complexes: recent progress in biomaterials and corrosion applications
- PMID: 35540133
- PMCID: PMC9081553
- DOI: 10.1039/c8ra01890a
Acyclic and cyclic imines and their metal complexes: recent progress in biomaterials and corrosion applications
Abstract
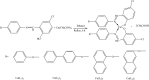
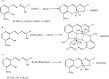
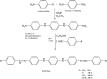
This review describes the contemporary development applications on scientific areas of acyclic and cyclic Schiff bases and their complexes with an emphasis on the author's contribution to the field. After a short historical introduction, this manuscript is divided into two main parts. In the first section, Schiff bases are reviewed for their biological activities including antibacterial, antifungal, antioxidant, cytotoxic, and enzymatic activities as well as their interaction with single-stranded-DNA which have shown remarkable activities in each region of research. The second part deals with the corrosion of metal and its alloys in corrosive environments and their organic inhibitors. The main section of this part is to investigate the different chemical structures for Schiff bases used in such aggressive solution to protect metals. Knowing the maximum corrosion efficiency or bioactivity of a specific chemical structure in a specific application environment is helpful for choosing the most appropriate compound.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
























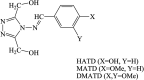


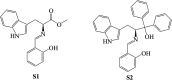






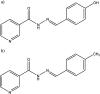









References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

