Synthesis of non-toxic, biocompatible, and colloidal stable silver nanoparticle using egg-white protein as capping and reducing agents for sustainable antibacterial application
- PMID: 35540173
- PMCID: PMC9081624
- DOI: 10.1039/c8ra03649g
Synthesis of non-toxic, biocompatible, and colloidal stable silver nanoparticle using egg-white protein as capping and reducing agents for sustainable antibacterial application
Erratum in
-
Correction: Synthesis of non-toxic, biocompatible, and colloidal stable silver nanoparticle using egg-white protein as capping and reducing agents for sustainable antibacterial application.RSC Adv. 2020 Nov 26;10(70):42960. doi: 10.1039/d0ra90124e. eCollection 2020 Nov 23. RSC Adv. 2020. PMID: 35532414 Free PMC article.
Retraction in
-
Retraction: Synthesis of non-toxic, biocompatible, and colloidal stable silver nanoparticle using egg-white protein as capping and reducing agents for sustainable antibacterial application.RSC Adv. 2022 Jun 17;12(28):18040. doi: 10.1039/d2ra90063g. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35800302 Free PMC article.
Abstract
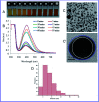
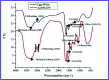
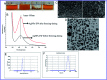
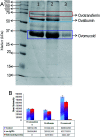
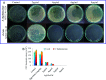
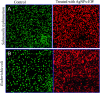
For nearly a decade, silver nanoparticles (AgNPs) have been the most prevalent commercial nanomaterials products widely used in different biomedical applications due to their broad-spectrum antimicrobial activity. However, their poor long-term stability in different environments, namely, pH, ionic strength, and temperature, and cytotoxicity toward mammalian cells has restricted their more extensive applications. Hence, there is urgent need to develop highly biocompatible, non-toxic, and stable silver nanoparticles for wide-ranging environments and applications. In the present study, a simple, sustainable, cost-effective and green method has been developed to prepare highly stable aqueous colloidal silver nanoparticles (AgNPs-EW) using the ovalbumin, ovotransferrin, and ovomucoid of egg-white as reducing and capping agents accomplished under the irradiation of direct sunlight. Then, we evaluated the effects of freezing-drying (lyophilization) and freeze-thaw cycles on the stability of AgNPs-EW in aqueous solution under visual inspection, transmission electron microscopy, and absorbance spectroscopy. In addition, we studied the antibacterial activity against Salmonella typhimurium and Escherichia coli, carried out biocompatibility studies on chicken blood, and tested acute, chronic toxicity in Drosophila melanogaster. The results suggest that AgNPs-EW did not aggregate upon freeze-thawing and lyophilization, thus exhibiting remarkable stability. The antibacterial activity results showed that the AgNPs-EW had the highest antibacterial activity, and the minimum inhibitory concentration (MIC) of AgNPs-EW for E. Coli and S. typhimurium were 4 and 6 μg ml-1, respectively. The biocompatibility study revealed that the AgNPs-EW did not induce any hemolytic effect or structural damage to the cell membranes of chicken erythrocytes up to a concentration of 12 μg ml-1. Similarly, no acute and chronic toxicity was observed on melanization, fecundity, hatchability, viability, and the duration of development in the 1st generation of Drosophila melanogaster at the concentration range of 10 mg L-1 to 100 mg L-1 of AgNPs-EW, and all the flies completed their full developmental cycle. Therefore, the present study successfully demonstrated the green and sustainable preparation of non-toxic AgNPs-EW having good biocompatibility, enhanced colloidal stability, and antibacterial activity. Hence, the synthesized AgNPs-EW could be used for the development of an antimicrobial formulation for controlling microbial infection.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All the authors declare that there are no conflicts of interest.
Figures




References
-
- Le Ouay B. Stellacci F. Nano Today. 2015;10:339–354. doi: 10.1016/j.nantod.2015.04.002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

