The synergetic effect of a structure-engineered mesoporous SiO2-ZnO composite for doxycycline adsorption
- PMID: 35540193
- PMCID: PMC9075987
- DOI: 10.1039/c9ra08106b
The synergetic effect of a structure-engineered mesoporous SiO2-ZnO composite for doxycycline adsorption
Abstract
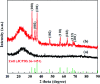
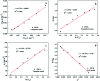
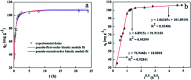
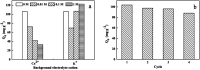
The design and synthesis of an efficient adsorbent for antibiotics-based pollutants is challenging due to the unique physicochemical properties of antibiotics. The development of a mesoporous SiO2-ZnO composite is a novel way to achieve excellent adsorption efficiency for doxycycline hydrochloride (DOX) in aqueous solutions due to the engineered highly open mesoporous structure and the ZnO-modified framework. Unlike the traditional method of obtaining mesoporous composites by post-synthesis techniques, the novel one-step method developed in this study is both effective and environment-friendly. The adsorption mechanism based on the novel synergetic effect between SiO2 and ZnO was demonstrated through several experiments. SiO2 led to the creation of a 3D open framework structure that provides sufficient space and rapid transport channels for adsorption, ensuring rapid adsorption kinetics. A higher number of active sites and enhanced affinity of the contaminants are provided by ZnO, ensuring high adsorption capacity. The mesoporous SiO2-ZnO could be easily regenerated without a significant decrease in its adsorption efficiency. These results indicate that the developed strategy afforded a simple approach for synthesizing the novel mesoporous composites, and that mesoporous SiO2-ZnO is a possible alternative adsorbent for the removal of DOX from wastewater.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- An H. J. Bhadra B. N. Khan N. A. Jhung S. H. Adsorptive removal of wide range of pharmaceutical and personal care products from water by using metal azolate framework-6-derived porous carbon. Chem. Eng. J. 2018;343:447–454. doi: 10.1016/j.cej.2018.03.025. - DOI
-
- O'Brien J. W. Banks A. P. Novic A. J. Mueller J. F. Jiang G. Ort C. Eaglesham G. Yuan Z. Thai P. K. Impact of in-Sewer Degradation of Pharmaceutical and Personal Care Products (PPCPs) Population Markers on a Population Model. Environ. Sci. Technol. 2017;51:3816–3823. doi: 10.1021/acs.est.6b02755. - DOI - PubMed
LinkOut - more resources
Full Text Sources

