Panax notoginseng saponins radiosensitize colorectal cancer cells by regulating the SNHG6/miR-137 axis
- PMID: 35540209
- PMCID: PMC9075843
- DOI: 10.1039/c9ra07622k
Panax notoginseng saponins radiosensitize colorectal cancer cells by regulating the SNHG6/miR-137 axis
Abstract
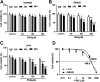
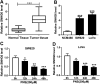
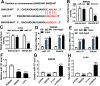
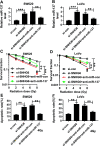
Panax notoginseng saponins (PNS) have recently attracted great attention for their anti-cancer activity in colorectal cancer (CRC). The aim of this study was to explore the functional role and underlying mechanisms of PNS on CRC radiosensitivity. Cell viability was assessed by a Cell Counting kit-8 assay. Cell survival and apoptosis were determined using colony formation assay and flow cytometry, respectively. Quantitative real-time PCR was used to quantify the levels of SNHG6 and miR-137. The targeted correlation between SNHG6 and miR-137 was validated by dual-luciferase reporter and RNA immunoprecipitation assays. Our data supported that PNS weakened the viability of CRC cells. Moreover, PNS promoted the radiosensitivity of CRC cells. Mechanistically, PNS enhanced CRC cell radiosensitivity by upregulating SNHG6. SNHG6 directly targeted miR-137 and inhibited miR-137 expression. MiR-137 was involved in the regulatory effect of SNHG6 on CRC cell radiosensitivity. Furthermore, PNS increased miR-137 expression through SNHG6 in CRC cells. Our study suggested that PNS promoted radiosensitivity in CRC cells at least partly through regulating the SNHG6/miR-137 axis, providing a novel understanding of the anti-cancer mechanism of PNS in CRC.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest regarding the publication of this paper.
Figures



Similar articles
-
Long non-coding RNA SNHG6 increases JAK2 expression by targeting the miR-181 family to promote colorectal cancer cell proliferation.J Gene Med. 2020 Dec;22(12):e3262. doi: 10.1002/jgm.3262. Epub 2020 Sep 21. J Gene Med. 2020. PMID: 32840014
-
lncRNA SNHG6 regulates EZH2 expression by sponging miR-26a/b and miR-214 in colorectal cancer.J Hematol Oncol. 2019 Jan 9;12(1):3. doi: 10.1186/s13045-018-0690-5. J Hematol Oncol. 2019. PMID: 30626446 Free PMC article.
-
LncRNA SNHG6 promotes the migration, invasion, and epithelial-mesenchymal transition of colorectal cancer cells by miR-26a/EZH2 axis.Onco Targets Ther. 2019 May 2;12:3349-3360. doi: 10.2147/OTT.S197433. eCollection 2019. Onco Targets Ther. 2019. PMID: 31118686 Free PMC article.
-
Knockdown of circ_0005615 enhances the radiosensitivity of colorectal cancer by regulating the miR-665/NOTCH1 axis.Open Med (Wars). 2023 May 9;18(1):20230678. doi: 10.1515/med-2023-0678. eCollection 2023. Open Med (Wars). 2023. PMID: 37727322 Free PMC article.
-
Long noncoding RNA SNHG6 functions as a competing endogenous RNA by sponging miR-181a-5p to regulate E2F5 expression in colorectal cancer.Cancer Manag Res. 2019 Jan 10;11:611-624. doi: 10.2147/CMAR.S182719. eCollection 2019. Cancer Manag Res. 2019. Retraction in: Cancer Manag Res. 2023 May 18;15:433-434. doi: 10.2147/CMAR.S421716. PMID: 30666158 Free PMC article. Retracted.
Cited by
-
LncRNA SNHG6 role in clinicopathological parameters in cancers.Eur J Med Res. 2023 Sep 21;28(1):363. doi: 10.1186/s40001-023-01358-2. Eur J Med Res. 2023. PMID: 37735423 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

