In situ reduction of chloroauric acid (HAuCl4) for generation of catalytic Au nanoparticle embedded triazine based covalent organic polymer networks
- PMID: 35540227
- PMCID: PMC9075937
- DOI: 10.1039/c9ra08822a
In situ reduction of chloroauric acid (HAuCl4) for generation of catalytic Au nanoparticle embedded triazine based covalent organic polymer networks
Abstract
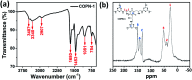
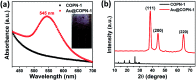
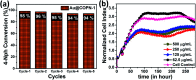
Covalent-organic polymer networks (COPNs) have been used as catalyst supports due to their stable and favorable structure. Herein, a simple synthetic route was applied to generate Au@COPN-1 hybrids via in situ reduction of gold ions with no additional reducing agent. Synthesized novel COPN-1 was mixed with different concentrations of HAuCl4 which resulted in Au@COPN-1 with varying sizes of Au nanoparticles in a controlled manner. The microstructural and morphological features of COPN-1 and Au@COPN-1 were characterized in detail using FT-IR, C-NMR, elemental analysis, UV-Vis, XRD, TEM, BET, and TGA. It is noteworthy that the red-shifted LSPR peaks of Au nanoparticles produced with increasing concentrations of HAuCl4 indicated an increase in the particle size of the Au nanoparticles as justified by TEM images. The optimum catalytic activity of Au@COPN-1 was obtained when 4.6 × 10-3 mM HAuCl4 was used, which led to the complete reduction of 4-nitrophenol within 16 minutes with excellent recyclability for more than 5 catalytic cycles, giving yields over 94%. Moreover, the non-aggregation of nanoparticles in the reused catalyst further confirmed the stability of the prepared catalysts. Consequently, these results indicated that in situ synthesis of AuNPs inside the COPN-1 matrix produces a promising catalyst platform for the reduction of aromatic nitro compounds, for example, for the degradation of one of the most common persistent organic pollutants 4-nitrophenol, as shown here. In addition, the Au@COPN-1 hybrid system showed good biocompatibility at appropriate doses confirmed by a dynamic real-time cell analysis system which can be used in various medical applications, such as drug delivery, in the future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Eco-friendly microwave-assisted green and rapid synthesis of well-stabilized gold and core-shell silver-gold nanoparticles.Carbohydr Polym. 2016 Jan 20;136:1128-36. doi: 10.1016/j.carbpol.2015.10.003. Epub 2015 Oct 9. Carbohydr Polym. 2016. PMID: 26572455
-
A Hexagonal Covalent Porphyrin Framework as an Efficient Support for Gold Nanoparticles toward Catalytic Reduction of 4-Nitrophenol.Chemistry. 2016 Nov 14;22(47):17029-17036. doi: 10.1002/chem.201603212. Epub 2016 Oct 13. Chemistry. 2016. PMID: 27734535
-
Solid-solid synthesis of covalent organic framework as a support for growth of controllable ultrafine Au nanoparticles.Sci Total Environ. 2022 Aug 20;835:155423. doi: 10.1016/j.scitotenv.2022.155423. Epub 2022 Apr 22. Sci Total Environ. 2022. PMID: 35469885
-
Eco-friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4-nitrophenol.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Jul 15;128:357-62. doi: 10.1016/j.saa.2014.02.083. Epub 2014 Mar 12. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24681320
-
Highly stable covalent organic framework-Au nanoparticles hybrids for enhanced activity for nitrophenol reduction.Chem Commun (Camb). 2014 Mar 25;50(24):3169-72. doi: 10.1039/c3cc49176e. Epub 2014 Feb 11. Chem Commun (Camb). 2014. PMID: 24519675
Cited by
-
Tumor-derived covalent organic framework nanozymes for targeted chemo-photothermal combination therapy.iScience. 2023 Jul 16;26(8):107348. doi: 10.1016/j.isci.2023.107348. eCollection 2023 Aug 18. iScience. 2023. PMID: 37554442 Free PMC article.
-
Green Synthesis of Homogeneous Gold Nanoparticles Using Sargassum spp. Extracts and Their Enhanced Catalytic Activity for Organic Dyes.Toxics. 2021 Oct 28;9(11):280. doi: 10.3390/toxics9110280. Toxics. 2021. PMID: 34822671 Free PMC article.
-
Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation.Molecules. 2022 Sep 7;27(18):5776. doi: 10.3390/molecules27185776. Molecules. 2022. PMID: 36144512 Free PMC article.
-
Preparation and catalytic properties of poly(methyl methacrylate)-supported Pd0 obtained from room-temperature, dark reduction of ionic aggregates of the unstable Pd2+ solution ionomer.RSC Adv. 2020 Nov 27;10(70):43175-43186. doi: 10.1039/d0ra08653c. eCollection 2020 Nov 23. RSC Adv. 2020. PMID: 35514939 Free PMC article.
-
Gold nanoparticles synthesis and immobilization by atmospheric pressure DBD plasma torch method.Nanoscale Adv. 2023 Apr 13;5(9):2573-2582. doi: 10.1039/d3na00007a. eCollection 2023 May 2. Nanoscale Adv. 2023. PMID: 37143807 Free PMC article.
References
-
- Liu J. Fu Y. Fu X. Li Y. Liang D. Song Y. Pan C. Yu G. Xiao X. RSC Adv. 2016;6:20834–20842. doi: 10.1039/C6RA01044J. - DOI
-
- Qu D. Liu F. Yu J. Xie W. Xu Q. Li X. Huang Y. Appl. Phys. Lett. 2011;98:113119. doi: 10.1063/1.3559225. - DOI
-
- Subair R. Tripathi B. P. Formanek P. Simon F. Uhlmann P. Stamm M. Chem. Eng. J. 2016;295:358–369. doi: 10.1016/j.cej.2016.02.105. - DOI
LinkOut - more resources
Full Text Sources

