Solid-state dye-sensitized solar cells based on Zn1- x Sn x O nanocomposite photoanodes
- PMID: 35540245
- PMCID: PMC9081753
- DOI: 10.1039/c8ra02852d
Solid-state dye-sensitized solar cells based on Zn1- x Sn x O nanocomposite photoanodes
Expression of concern in
-
Expression of Concern: Solid-state dye-sensitized solar cells based on Zn1-xSnxO nanocomposite photoanodes.RSC Adv. 2023 Nov 8;13(47):32927. doi: 10.1039/d3ra90109b. eCollection 2023 Nov 7. RSC Adv. 2023. PMID: 38025877 Free PMC article.
Abstract
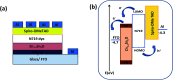
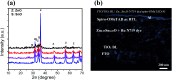
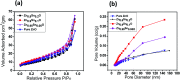
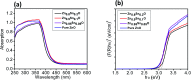
Solid-state dye-sensitized solar cells (ss-DSSCs) comprising Sn2+-substituted ZnO nanopowder were purposefully tailored via a co-precipitation method. The solar cells assembled in this work were sensitized with N719 ruthenium dye and insinuated with spiro-OMeTAD as a solid hole transport layer (HTL). Evidently, significant enhancement in cell efficiency was accomplished with Sn2+ ions-substituted ZnO photoelectrodes by maintaining the weight ratio of SnO at 5%. The overall power conversion efficiency was improved from 3.0% for the cell with pure ZnO to 4.3% for the cell with 5% SnO substitution. The improvement in the cell efficiency with Sn2+-substituted ZnO photoelectrodes is attributed to the considerably large surface area of the nanopowders for dye adsorption, efficient charge separation and the suppression of charge recombination provided by SnO. Furthermore, the energy distinction between the conduction band edges of SnO and ZnO implied a type II band alignment. Moreover, the durability as well as the stability of 15 assembled cells were studied to show the outstanding long-term stability of the devices made of Sn2+ ion substituted ZnO, and the PCE of each cell remained stable and ∼96% of its primary value was retained for up to 100 h. Subsequently, the efficacy was drastically reduced to ∼35% after 250 h of storage.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Omar A. Abdullah H. Renewable Sustainable Energy Rev. 2014;31:149–157. doi: 10.1016/j.rser.2013.11.031. - DOI
-
- O'Regan B. Grätzel M. Nature. 1991;335:737–740. doi: 10.1038/353737a0. - DOI
-
- Wu W.-Q. Chen D. Cheng Y.-B. Caruso R. A. Sustainable Energy Fuels. 2017;1:1960–1967. doi: 10.1039/C7SE00377C. - DOI
-
- Gong J. Liang J. Sumathy K. Renewable Sustainable Energy Rev. 2012;16:5848–5860. doi: 10.1016/j.rser.2012.04.044. - DOI
LinkOut - more resources
Full Text Sources

