Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer's disease
- PMID: 35540246
- PMCID: PMC9081849
- DOI: 10.1039/c8ra03620a
Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer's disease
Abstract
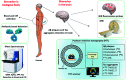
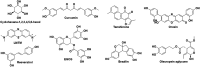
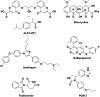
Alzheimer's disease (AD) is the most prevalent, progressive and multifaceted neurodegenerative disorder associated with cognition, memory and behavioural impairments. There is no approved diagnosis or cure for AD, and it affects both developed and developing countries and causes a significant social and economic burden. Extracellular senile plaques of amyloid beta (Aβ) and intracellular neurofibrillary tangles of phosphorylated Tau (pTau) in the brain are considered to be the pathophysiological hallmarks of AD. In an attempt to explain the complexity and multifactorial nature of AD, various hypotheses (Aβ aggregation, Tau aggregation, metal dyshomeostasis, oxidative stress, cholinergic dysfunction, inflammation and downregulation of autophagy) based on pathophysiological changes that occur during the onset and progression of AD have been proposed. However, none of the hypotheses is capable of independently explaining the pathological conditions observed in AD. The complex and multifaceted pathophysiological nature of AD has hampered the identification and validation of effective biomarkers for early diagnosis and the development of disease-modifying therapies. Nevertheless, the amyloid hypothesis is the most widely accepted and is closely correlated with disease symptoms of AD that encompass all the disease hypotheses. Therefore, amyloid plaques are ideal biomarkers for the development of an early diagnosis of AD. Similarly, the formation of amyloid plaques can also serve as a target for the design of therapeutic tools via an inclusive approach that considers multiple disease pathways involved in AD. Our review article briefly introduces pathophysiological factors involved in AD using interdependent but diverse hypotheses. Recent advances in the development of effective molecular tools and techniques for diagnostic and therapeutic interventions in AD, especially those in the advanced stages (clinical trials) of development, are given special consideration. In addition, contributions from our laboratory to the development of selective molecular tools for diagnostic and therapeutic interventions that target multifaceted toxicity in AD are also covered. In summary, we discuss diverse aspects of molecular mechanisms that underlie the pathogenesis of multifactorial AD, current progress and possible bottlenecks that have hampered the development of early diagnostic tools and effective drugs. Challenges and future prospects include the integration of various disease pathways for the successful development of an early diagnosis and effective drugs for the treatment of AD.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

