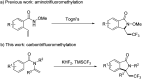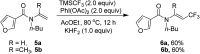Synthesis of trifluoromethyl-containing isoindolinones from tertiary enamides via a cascade radical addition and cyclization process
- PMID: 35540298
- PMCID: PMC9081866
- DOI: 10.1039/c8ra03696a
Synthesis of trifluoromethyl-containing isoindolinones from tertiary enamides via a cascade radical addition and cyclization process
Abstract
A radical trifluoromethylation reaction of tertiary enamides was investigated and trifluoromethyl-containing isoindolinones were prepared under mild conditions. Using TMSCF3 as a radical source, PhI(OAc)2 as an oxidant and KHF2 as an additive, tertiary enamides were converted to isoindolinones via a cascade addition and cyclization process in moderate to good yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
C(sp2)-H Trifluoromethylation of enamides using TMSCF3: access to trifluoromethylated isoindolinones, isoquinolinones, 2-pyridinones and other heterocycles.Chem Commun (Camb). 2018 Sep 18;54(75):10574-10577. doi: 10.1039/c8cc04907f. Chem Commun (Camb). 2018. PMID: 30156224
-
Metal- and additive-free β-C(sp2)-H decarboxylative alkylsulfonylation of enamides from phenyliodine(III) dicarboxylates and sulfur dioxide.Chem Commun (Camb). 2024 Aug 1;60(63):8212-8215. doi: 10.1039/d4cc02865a. Chem Commun (Camb). 2024. PMID: 39010756
-
Trichloroisocyanuric Acid Promoted Cascade Cyclization/Trifluoromethylation of Allylic Oximes: Synthesis of Trifluoromethylated Isoxazolines.Org Lett. 2017 Jan 20;19(2):376-379. doi: 10.1021/acs.orglett.6b03582. Epub 2017 Jan 9. Org Lett. 2017. PMID: 28067052
-
Copper-Mediated Domino Cyclization/Trifluoromethylation/Deprotection with TMSCF3: Synthesis of 4-(Trifluoromethyl)pyrazoles.Org Lett. 2017 Feb 3;19(3):658-661. doi: 10.1021/acs.orglett.6b03822. Epub 2017 Jan 12. Org Lett. 2017. PMID: 28080073
-
Controlling the regiochemistry of radical cyclizations.Chem Rec. 2006;6(1):23-31. doi: 10.1002/tcr.20069. Chem Rec. 2006. PMID: 16470801 Review.
References
-
-
For reviews, see:
- Wang J. Sanchez-Rosello M. Acena J. L. del Pozo C. Sorochinsky A. E. Fustero S. Soloshonok V. A. Liu H. Chem. Rev. 2014;114:2432. doi: 10.1021/cr4002879. - DOI - PubMed
- Gakh A. A. Shermolovich Y. Curr. Top. Med. Chem. 2014;14:952. doi: 10.2174/1568026614666140202210424. - DOI - PubMed
-
-
- Zheng Y. Ma J. A. Adv. Synth. Catal. 2010;352:2745. doi: 10.1002/adsc.201000545. - DOI
- Studer A. Angew. Chem., Int. Ed. 2012;51:8950. doi: 10.1002/anie.201202624. - DOI - PubMed
- Koike T. Akita M. Top. Catal. 2014;57:967. doi: 10.1007/s11244-014-0259-7. - DOI
- Honey M. A. Pasceri R. Lewis W. Moody C. J. J. Org. Chem. 2012;77:1396. doi: 10.1021/jo202201w. - DOI - PubMed
- Bouillon J. P. Ates C. Janousek Z. Viehe H. G. Tetrahedron Lett. 1993;34:5075. doi: 10.1016/S0040-4039(00)60679-2. - DOI
-
- Yu J. Yang H. Fu H. Adv. Synth. Catal. 2014;356:3669. doi: 10.1002/adsc.201400144. - DOI
-
- Lin J. S. Liu X. G. Zhu X. L. Tan B. Liu X. Y. J. Org. Chem. 2014;79:7084. doi: 10.1021/jo5012619. - DOI - PubMed
- Lin J. S. Xiong Y. P. Ma C. L. Zhao L. J. Tan B. Liu X. Y. Chem.–Eur. J. 2014;20:1332. doi: 10.1002/chem.201303387. - DOI - PubMed
- Zhang H. Y. Huo W. Ge C. Zhao J. Zhang Y. Synlett. 2017;28:962. doi: 10.1055/s-0036-1588400. - DOI
-
- Xu P. Xie J. Xue Q. Pan C. Cheng Y. Zhu C. Chem.–Eur. J. 2013;19:14039. doi: 10.1002/chem.201302407. - DOI - PubMed
- Egami H. Shimizu R. Sodeoka M. J. Fluorine Chem. 2013;152:51. doi: 10.1016/j.jfluchem.2013.03.009. - DOI
- An Y. Li Y. Wu J. Org. Chem. Front. 2016;3:570. doi: 10.1039/C6QO00055J. - DOI
- Kong W. Casimiro M. Merino E. Nevado C. J. Am. Chem. Soc. 2013;135:14480. doi: 10.1021/ja403954g. - DOI - PubMed
- Yang N. Li Z. Ye L. Tan B. Liu X. Y. Chem. Commun. 2016;52:9052–9055. doi: 10.1039/C6CC00364H. - DOI - PubMed
LinkOut - more resources
Full Text Sources