Design of triphasic poly(lactic- co-glycolic acid) nanoparticles containing a perfluorocarbon phase for biomedical applications
- PMID: 35540375
- PMCID: PMC9078287
- DOI: 10.1039/c7ra13062g
Design of triphasic poly(lactic- co-glycolic acid) nanoparticles containing a perfluorocarbon phase for biomedical applications
Abstract
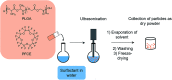
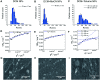
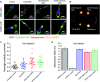
Poly(lactic-co-glycolic acid) (PLGA) particles are very widely used, particularly for drug delivery, including commercial clinical formulations. Adding perfluorocarbon (PFC) enables in vivo imaging and quantification of the PLGA particles through 19F NMR, MRS or MRI. PFCs are both hydrophobic and lipophobic at the same time. This property makes their encapsulation in particles challenging, as it requires the addition of a third immiscible phase during the emulsification process. Here we explore how different parameters affect the miniemulsion formation of particles loaded with perfluoro-15-crown-5-ether (PFCE). By changing the concentration of surfactant and type of solvent, we were able to control the radius of synthesized particles, between 85-200 nm. We assessed stability and release from the particles at different pH values, showing that hydrophobic agents are released from the particles by diffusion rather than degradation. With cell experiments, we show that primary human dendritic cells take up the particles without any apparent effect, including on cell migration. In summary, the control of synthesis conditions leads to particles with sufficient PFCE encapsulation, which are suitable for drug loading and cell labeling, and do not affect cell viability or functionality. Finally, these nanoparticles can be produced at GMP-grade for clinical use.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
-
- Fuchs A. V. Bapat A. P. Cowin G. J. Thurecht K. J. Switchable 19F MRI polymer theranostics: towards in situ quantifiable drug release. Polym Chem. 2017;8(34):5157–5166. doi: 10.1039/C7PY00345E. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

